Overview of Research Performance Progress Report (RPPR)
What is the purpose?
Progress reports are required annually to document recipient accomplishments and compliance with terms of award. They describe scientific progress, identify significant changes, report on personnel, and describe plans for the subsequent budget period or year.
The Office of Management and Budget (OMB) has mandated the use of a federal-wide research performance progress report (RPPR) by all federal awarding agencies for submission of required annual, final, and interim performance reporting on grants and cooperative agreement awards to standardize recipient reporting on federally-funded research projects.
Main Screenshots
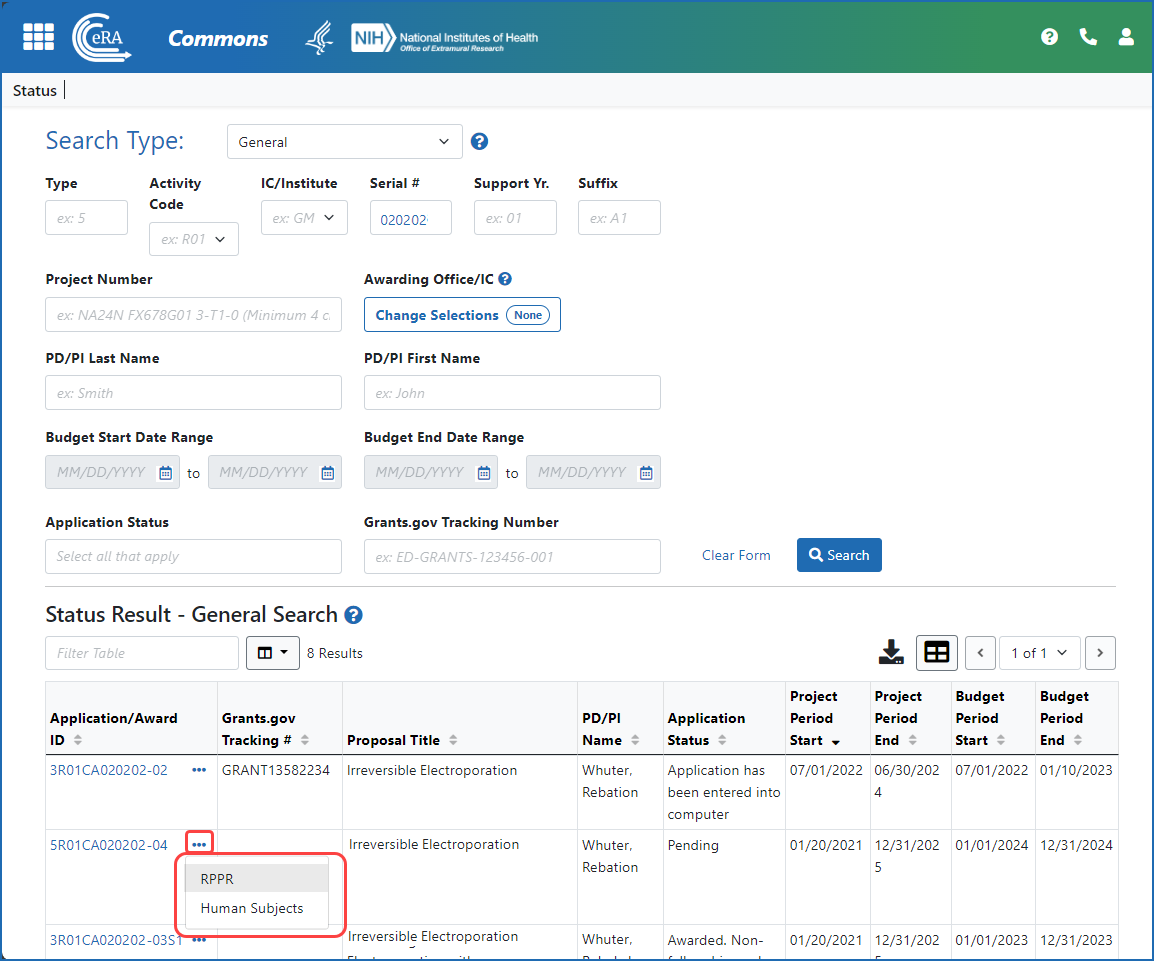
Figure 1: A signing official (SO) accesses an RPPR via the three-dot ellipsis menu in search results in the Status module.
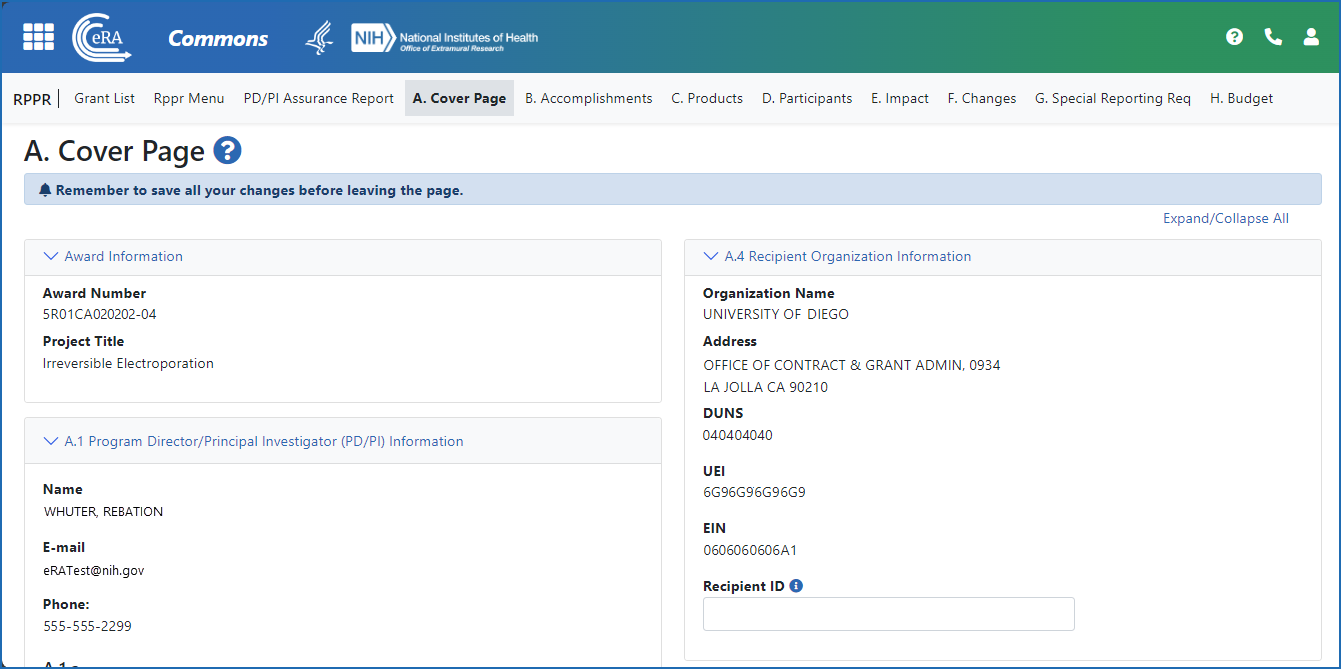
Figure 2: The Annual RPPR form and navigation tabs
The Use of RPPR
NIH requires use of the RPPR module in eRA Commons to submit all annual progress reports, as well as Final RPPR for award closeout, and the Interim RPPR when an awarded institution applies for a competitive renewal (Type II) application.
What are the benefits of RPPR?
Here is a list of the features and benefits of RPPR:
Because RPPR is integrated with eRA Commons, much of the information is pre-populated from NIH systems for the recipient, including PD/PI information, grant number, project title and period, performance sites, and personnel (except in the first year of RPPR use for progress reports not previously submitted through eRA Commons). The PD/PI’s publications, if linked to his/her Commons account from MY NCBI (as is required for NIH), are displayed for easy association with the progress report.
- RPPR addresses NIH specific policies such as required human subjects education, inclusion enrollment reporting, use of human embryonic stem cells, etc.
- The format of the report is user friendly. Users answer questions using a checkbox, by entering text or uploading a PDF, or selecting ‘Nothing to Report.’
- A request can be made to recipients for additional information for the progress report that can be submitted via eRA Commons.
- An institute can request additional material seeking clarification on an issue from a recipient, following submission of the progress report.
- An institute can also request verification that the recipient is in compliance with the NIH Public Access Policy, which requires recipients to make available to the public any publications that arose from federally-funded grants (within 12 months of publication).
- Other features of the RPPR include:
- A specific location to report award-related competitive revisions/administrative supplements.
- Automated indication of compliance with the Public Access Policy
- Other support is only required if there has been a change
- A link to the Notice of Award
Resources
Here are some helpful links:
- NIH Grants web page for RPPR
- "How to" information on the Research Performance Progress Reports (RPPR)




 eRA Intranet
eRA Intranet