Information and resources on how to submit the three variations of the Research Performance Progress Report can found on this page.
All progress reports for NIH grants must be submitted electronically using the Research Performance Progress Report (RPPR) module in eRA Commons (See Grants & Funding’s RPPR webpage for details). Progress reports document the recipient’s accomplishments and compliance with terms of award.
There are three types of RPPRs:
- Annual RPPR – Used to describe a grant’s scientific progress, identify significant changes, report on personnel, and describe plans for the subsequent budget period or year.
- Interim RPPR – Used when submitting a renewal (Type 2) application. If the Type 2 is not funded, the Interim RPPR will serve as the Final RPPR for the project. If the Type 2 is funded, the Interim RPPR will serve as the annual RPPR for the final year of the previous competitive segment. The data elements collected on the Interim RPPR are the same as for the Final RPPR, including project outcomes.
- Final RPPR – Used as part of the grant closeout process to submit project outcomes in addition to the information submitted on the annual RPPR, except budget and plans for the upcoming year.
Basic Tasks (step-by-step instructions from the online help)*
- For Program Directors/Principal Investigators to initiate an RPPR
- For Signing Officials to submit an RPPR in eRA Commons
- For Signing Officials to delegate submission of an RPPR
- Submitting Your Interim Research Performance Progress Report
- Submitting Your Final Research Performance Progress Report
* You must be logged into eRA Commons with appropriate role(s) to complete these activities.
Main Screenshots
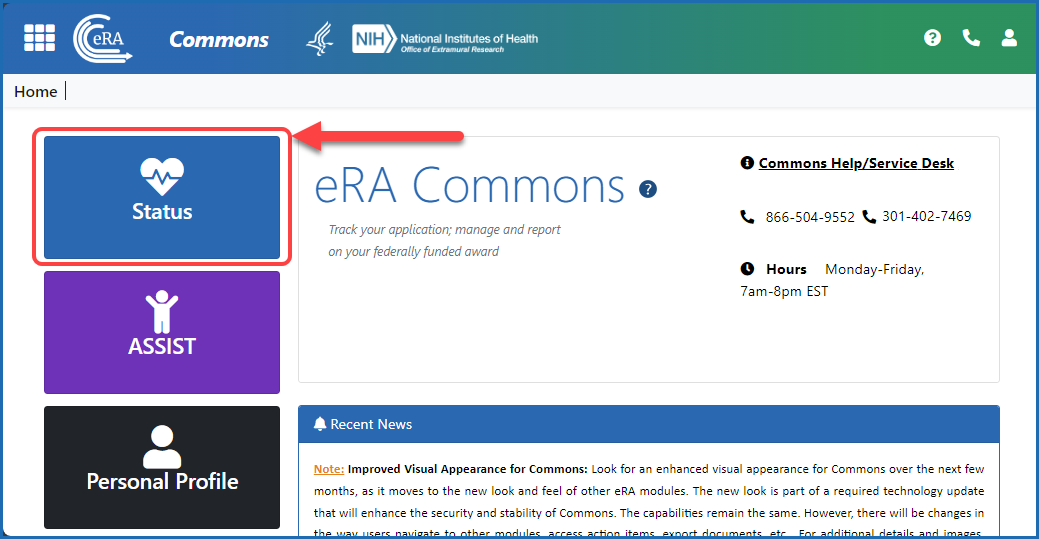
Figure 1: After logging in to eRA Commons, navigate to Status by clicking the Status button on the Home screen.
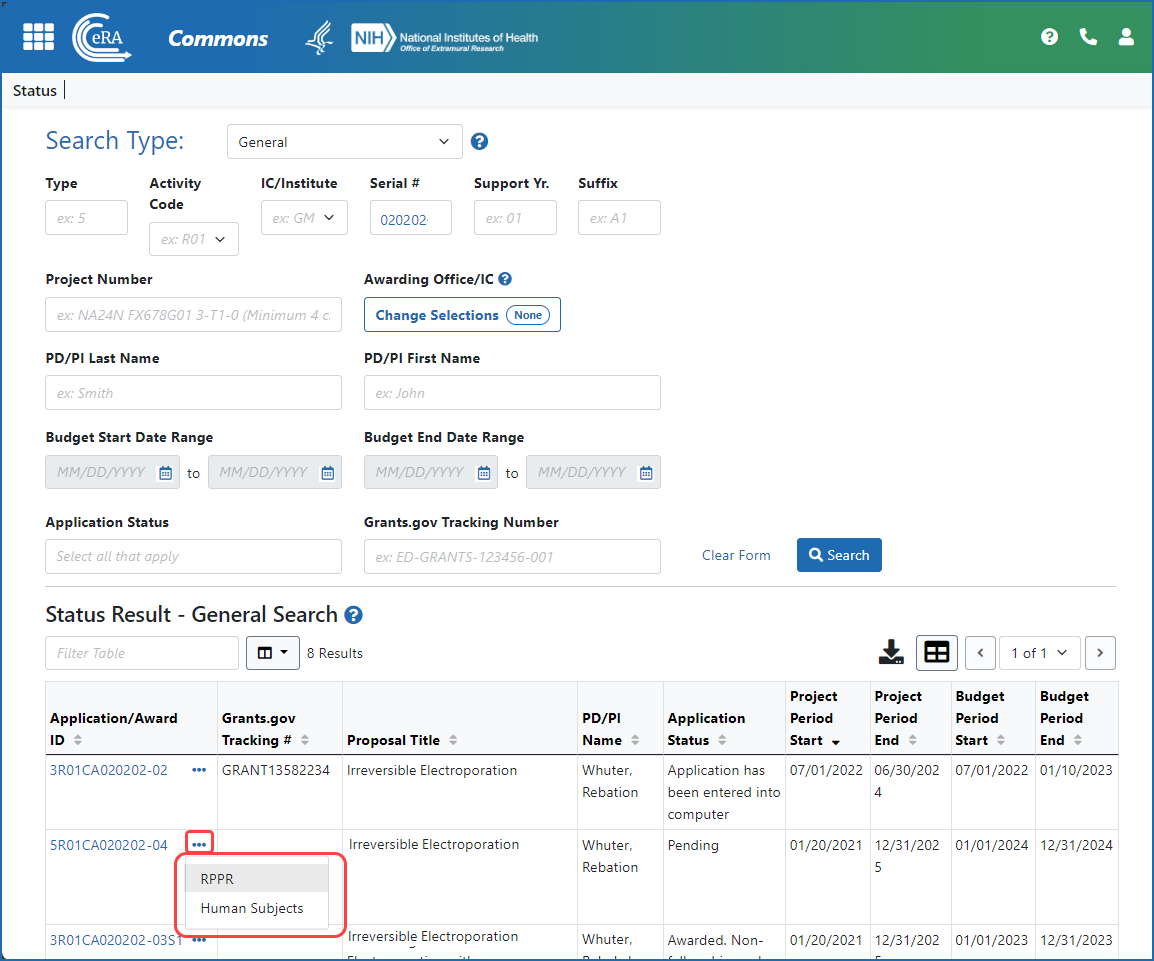
Figure 2: A signing official (SO) accesses an RPPR via the three-dot ellipsis menu in search results in the Status module.
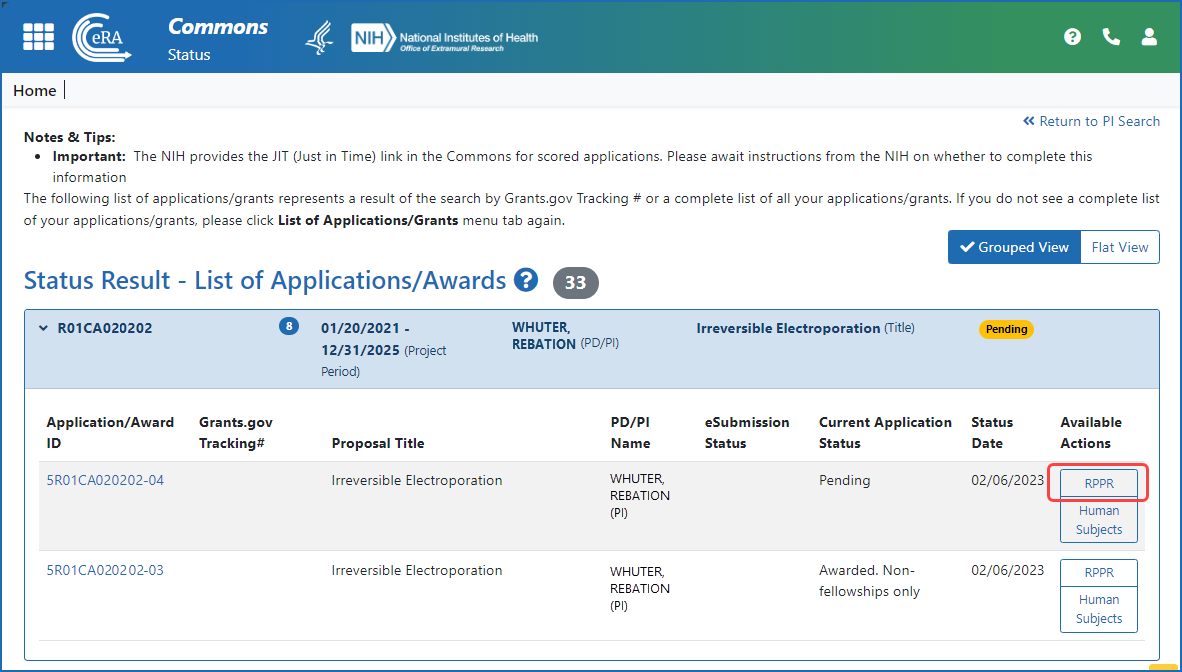
Figure 3: A principal investigator (PI) accesses an RPPR via the RPPR button in the Status module.
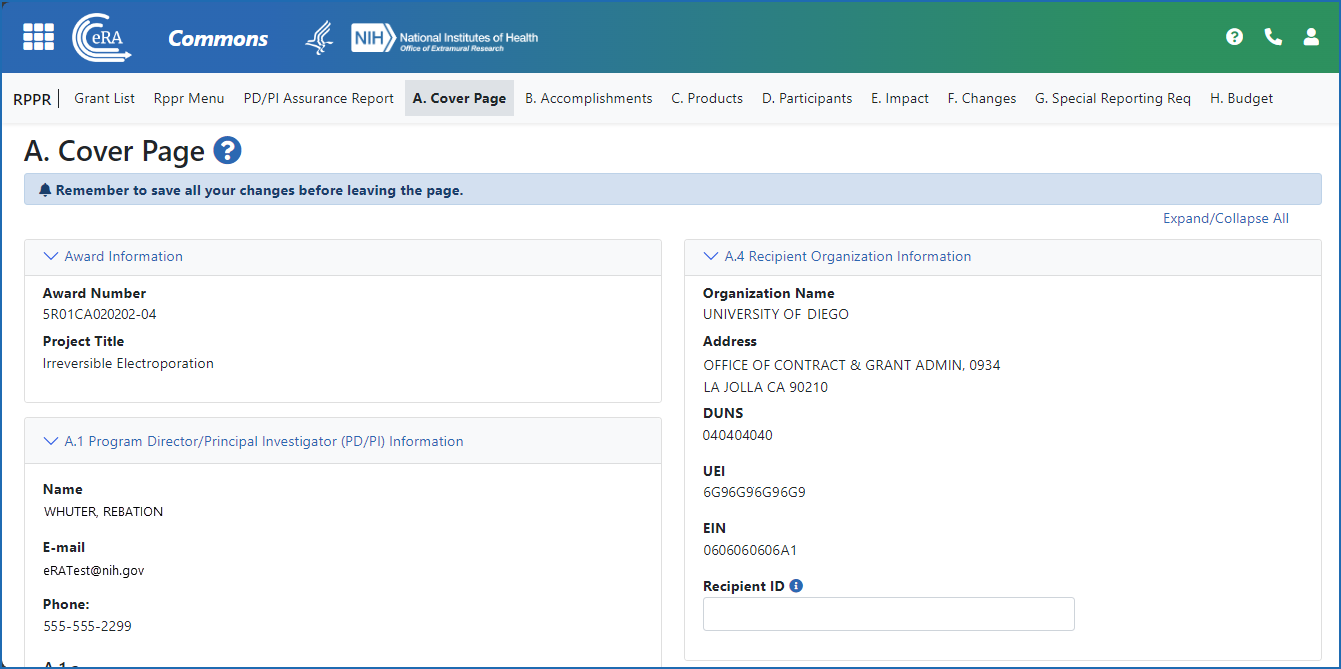
Figure 4: The Annual RPPR form and navigation tabs
Interim RPPR Scenarios
| Competing Renewal Application Status | Action |
| Not submitting a Competing Renewal application | Submit Final RPPR no later than 120 days from the project period end date |
| Submitting a Competing Renewal application* | Submit Interim RPPR no later than 120 days from the project period end date |
* If the competing renewal application is funded, the Interim RPPR is accepted as the Annual RPPR. If, however, the renewal is NOT funded, the Interim RPPR is accepted as the Final RPPR.
Additional Resources
- RPPR Instruction Guide
- RPPR Resources Page
- RPPR Webpage (Grants & Funding)
- Grants Closeout FAQs
- RPPR Training (webinar, slide presentations)
- RPPR Phase II Training for Grantees Webinar (Video) (November 2014)
- RPPR Phase II Training for Grantees Webinar Questions (Video) (November 2014)
- RPPR Phase II Training for Grantees (PowerPoint) (November 2014)
- RPPR: Who Can Do What? (PDF - 76KB) (September 2018)
- eRA Commons Roles & Privileges At a Glance (PDF - 25 KB)
Policy Links
- NIH Grants Policy Statement: RPPR
- NIH Grants Policy Statement: Final Progress Report
- NIH Guide Notice NOT-OD-24-175: Reminder: Reporting Data Management and Sharing (DMS) Plan Activities in RPPRs Submitted on or after October 1, 2024

- NIH Guide Notice NOT-OD-17-085: NIH Implementation of Final RPPR for SBIR/STTR
- NIH Guide Notice NOT-OD-17-037: NIH Implementation of the Interim RPPR
- NIH Guide Notice NOT-OD-17-022: NIH Implementation of Final RPPR
- NIH Guide Notice NOT-OD-21-110: Implementation of Changes to the Biographical Sketch and Other Support Format Page




 eRA Intranet
eRA Intranet