Steps for accessing and reporting inclusion data in the Human Subjects System (HSS) for both grants and R&D Contracts, along with related resources can be found on this page.
The HSS system enables grant recipients and awarded R&D contract vendors to electronically report and update their data on human subjects and clinical trials to NIH, and for NIH agency staff to monitor and manage the data.
Note: NIH R&D contract vendors have the ability to submit inclusion data on their awarded contracts through HSS, accessed through eRA Commons, effective for all new and existing contracts, if requested by an NIH staff member (See NOT-OD-24-128). For submissions, the vendor organization needs to be registered in eRA Commons and any principal investigator (PI) who will be entering information will need a Commons PI account that is associated with the vendor organization.
For grants, the HSS is automatically populated by human subjects and clinical trial data entered by the principal investigator (PI) on the Human Subjects and Clinical Trial Information form in applications submitted to NIH. This data is then made available to PIs and signing officials(SOs) for adding or editing studies through a Human Subjects link on the eRA Commons Status screen.
To submit inclusion enrollment data in a Research Performance Progress Report (RPPR), grant recipients may also access HSS via the Human Subjects link in Section G.4.b Inclusion Enrollment Data of the RPPR, rather than through Status. Note that contract vendors can only access HSS through the Status screen.
As a reminder, information in HSS covers the current project period.
Accessing HSS via eRA Commons
- SO: Status tab > General Search screen > Specific Award >Action column > Human Subjects Link
- PI: Status tab > Status — PI Search screen > Status Result — List of Applications/Awards screen > Specific Award >Action column > Human Subjects Link
- Both (for grants only): RPPR tab > Manage RPPR > Specific Grant > RPPR Menu screen > Edit button > Inclusion Section (G.4.b) > Human Subjects Link
Accessing Status*
Once logged in to eRA Commons, click on Status on the landing screen, or click on the Main menu in the upper left corner of the screen and select Status from the drop-down menu. See Figures 1 and 2.
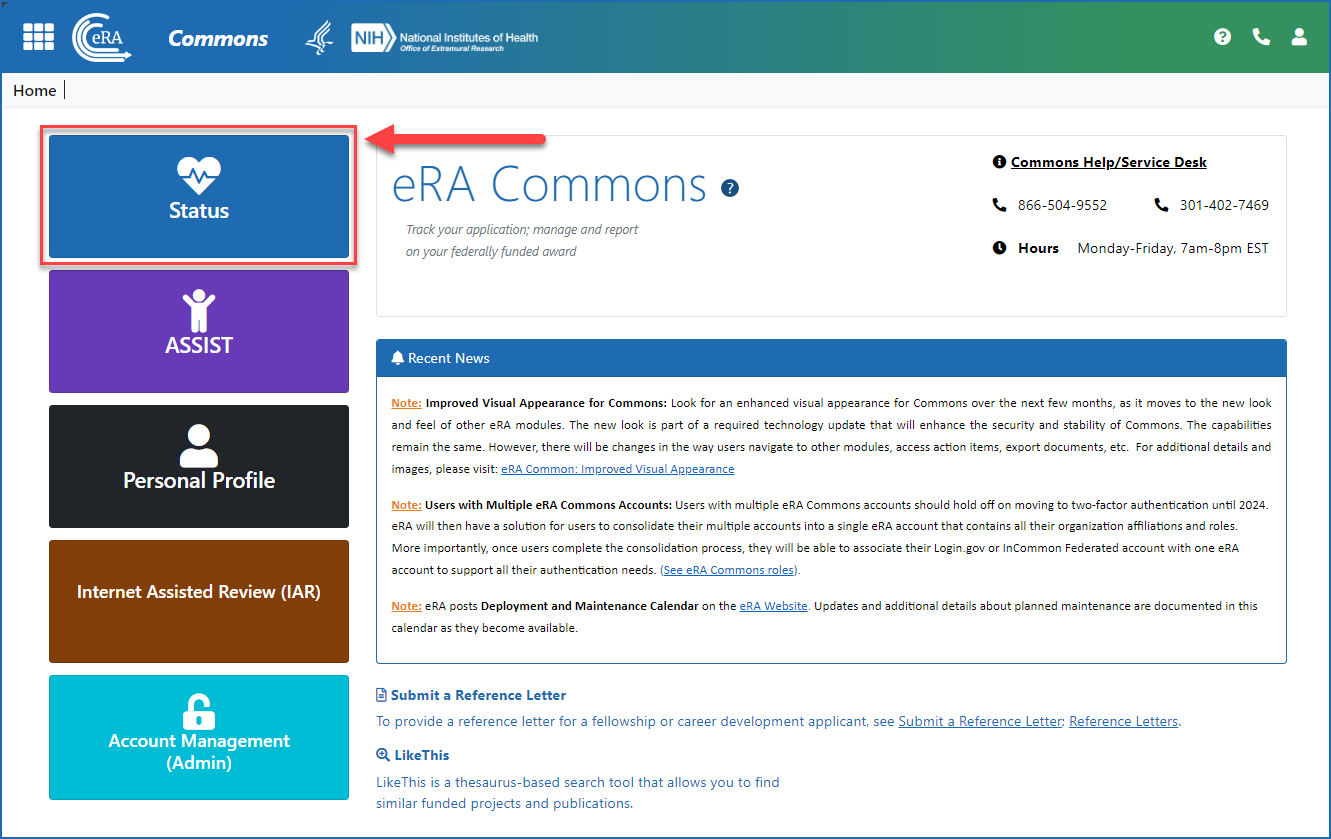
Figure 1: The Status button on the eRA Commons landing screen
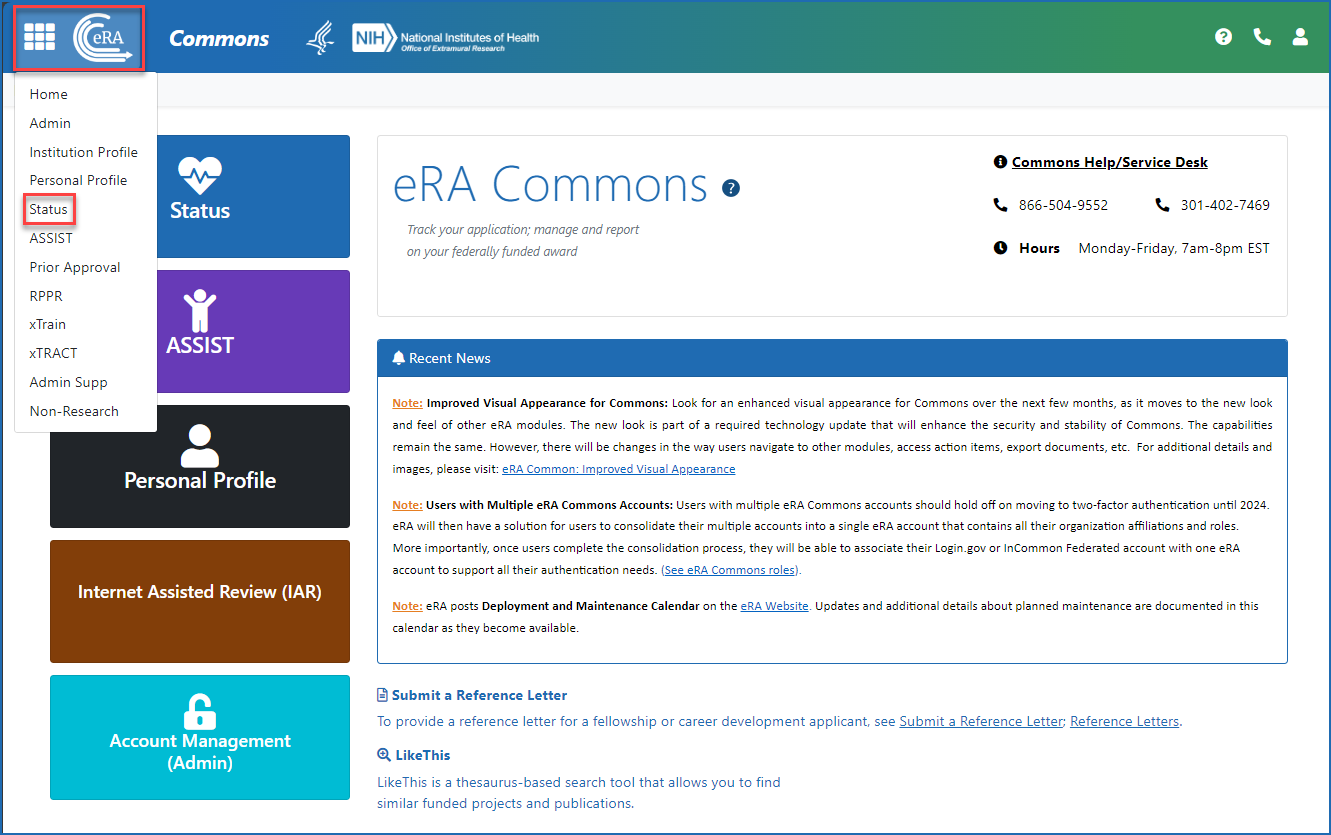
Figure 2: Accessing Status from the Main menu (apps icon or eRA logo) in the upper left of any eRA Commons screen
Basic Tasks (step-by-step instructions from the online help)*
* You must be logged into eRA Commons with appropriate role(s) to complete these activities.
Main Screenshots
Accessing the Human Subjects link from the Status screen (signing official)
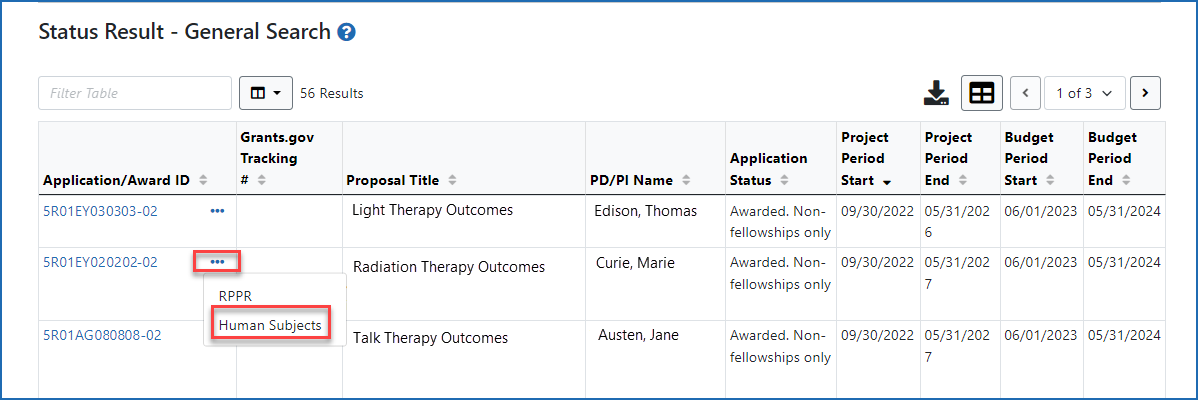
Figure 3: Accessing the Human Subjects link from the three-dot dropdown menu on the Status Results - General Search screen
Accessing the Human Subjects link from the Status screen (principal investigator)
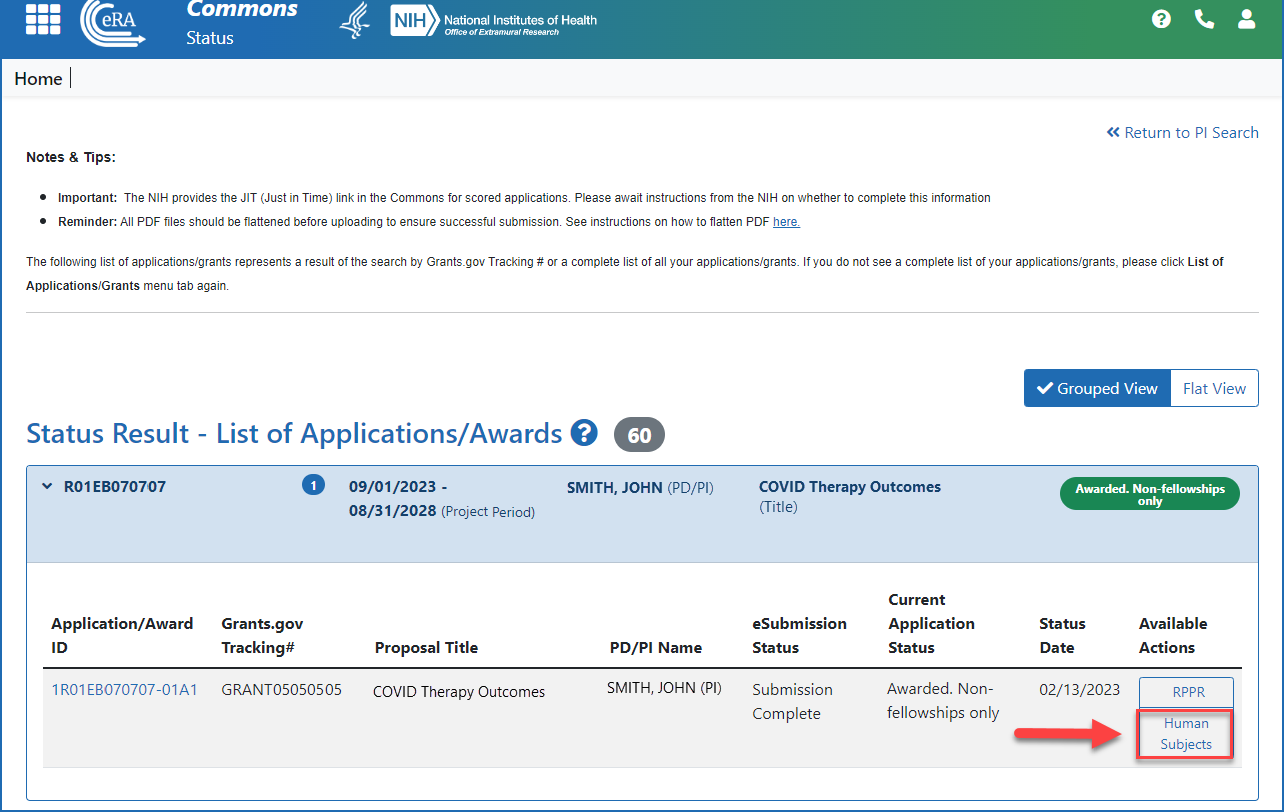
Figure 4: Accessing the Human Subjects link from the action column on the Status Results - List of Applications/Awards screen
Accessing the Human Subjects link from the RPPR (grant recipients)
Figure 5: Section G.4 of the RPPR showing the Human Subjects link
Additional Resources
- Human Subjects System Resource Page
- OER Human Subjects Research Page
- Human Subjects System video tutorials (6 videos)
- Tip Sheet: Using the Participant-level Data Template -- PDF; January 20, 2022
Participant-level Data Template -- CSV; February 7, 2025
For R&D Contract Vendors
- New eRA Human Subjects System Capabilities for Awarded NIH R&D Contract Vendors to Directly Enter Inclusion Data (NIH guide notice: NOT-OD-24-128) (June 6, 2024)
- Quick Start Guide for Contract Vendors: Updating Inclusion Data for Awarded R&D Contracts (June 3, 2024)
- Updating Inclusion Data for Awarded R&D Contracts (video tutorial) (May 16, 2024)
Policy Links
- NIH Grants Policy Statement: Human Subjects Protections
- NIH Guide Notice NOT-OD-18-179: Transition from Inclusion Management System to New Human Subjects System (HSS) as of June 9, 2018




 eRA Intranet
eRA Intranet