The Other Request type is used in different ways depending on the agency providing the award. Prior Approval "Other Request" can be used for both FDA and NIH awards. The Other Request type appears only for signing officials (SOs) and does not appear for program directors/principal investigators (PD/PIs).
For NIH awards, reach out to your grants management specialist (GMS) or program official (PO) for instructions on when to use the Other Request type. NIH awardees should NOT use the Prior Approval "Other Request" type to submit updates to their approved Data Management and Sharing (DMS) Plan. Instead, they should use the DMS Request type to submit an updated DMS Plan; see Prior Approval - DMS Request.
Basic Task (step-by-step instructions)
Main Screenshots
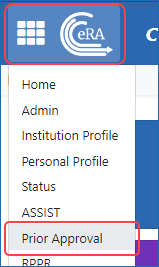
Figure 1: Access Prior Approval from the Main menu/eRA logo in upper left of eRA Commons
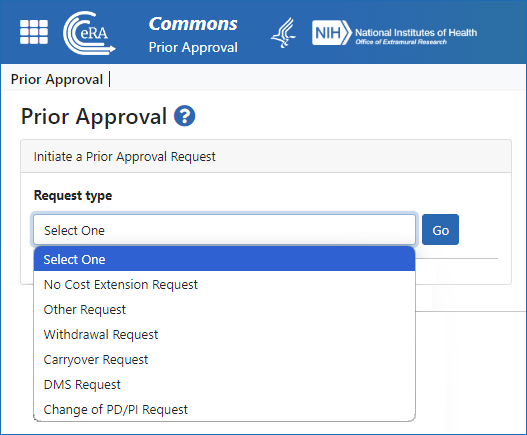
Figure 2: The Prior Approval landing screen, showing the Request Types dropdown
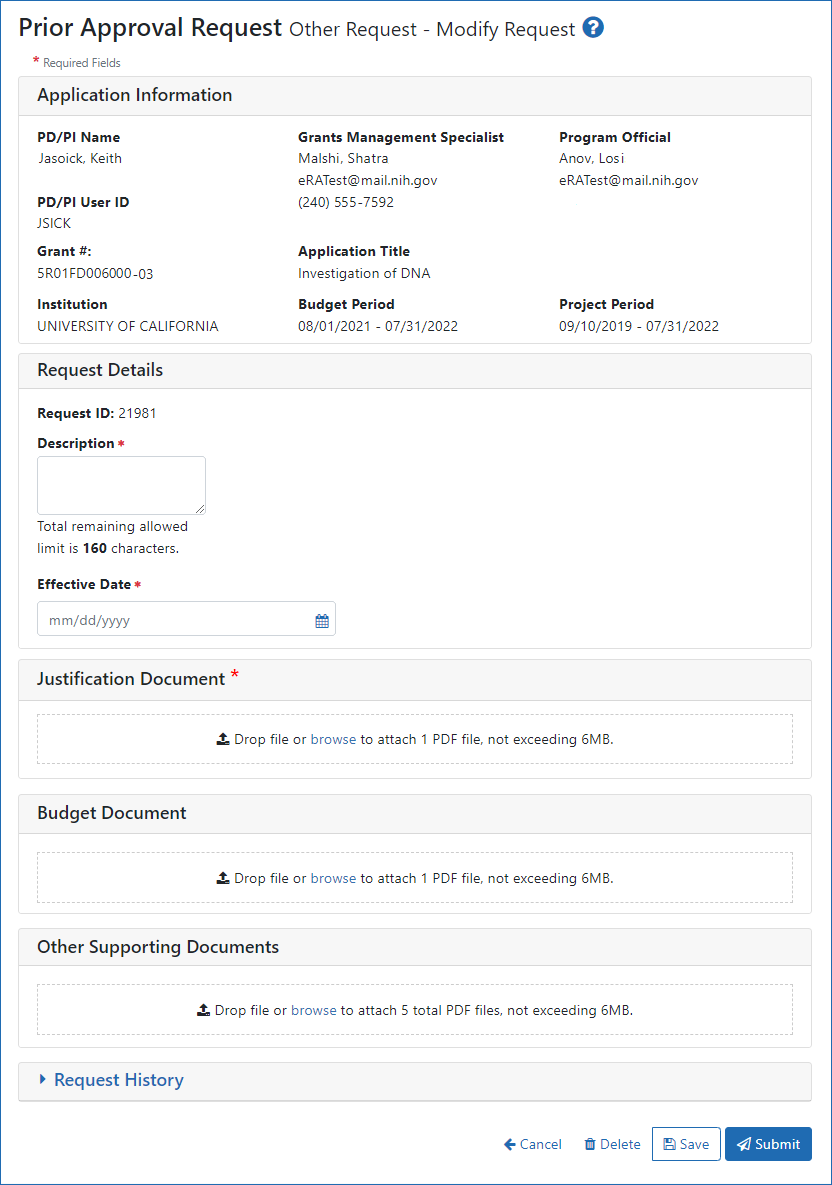
Figure 3: The Other Request screen, accessed through the Prior Approval module
Resources
- Prior Approval Module (online help)




 eRA Intranet
eRA Intranet