Jan 30, 2019
ASSIST Application Previews Prior to Dec. 12 To Be Removed
ASSIST users,
Last December we made some technology changes to how ASSIST manages uploaded attachments and generates application and form previews. We migrated all your uploaded attachments to the new technology and moved new application and form previews to the new technology.
On February 27, 2019, we will remove access to any application previews generated in ASSIST prior to December 12, 2018. You can generate a fresh preview at any time. Individual files uploaded in ASSIST and any submitted and assembled application images accessed in eRA Commons will remain in place.
Jan 23, 2019
New Validations for HSS Form When Completing an RPPR
A Human Subjects System (HSS) release on Thursday, January 24, 2019, will introduce the following validations and warning messages for Research Performance Progress Reports (RPPR). These validations are for Section 6 and other fields on the Human Subjects Clinical Trials form.
Scenario RPPR Validation Study’s inclusion Monitoring code is marked as Yes, but there is no planned enrollment data and the study is not an existing dataset or delayed onset. Warning message:
"Planned counts are required to be greater than zero for Inclusion Enrollment Reports <IER#, IER#> under Study<Study#>, Inclusion Enrollment Reports<IER#,IER#> under Study <Study #>. Please click on the Human Subjects link in G.4 to update the Inclusion Enrollment Report(s) in Section 2 of the Human Subjects and Clinical Trials Information form.
Enrollment of first participant was more than 21 days ago, but a Clinicaltrials.gov identifier (NCT) has not been provided. Warning message:
"Enrollment for <study title> has begun but no Clinicaltrials.gov Identification Number (NCT) has been provided. Please complete Clinicaltrials.gov registration and use the Human Subjects link in G.4 to add the NCT number in the Human Subjects and Clinical Trial Information Form item 1.5."
The actual Primary Completion Date is more than 12 months ago and results have not been reported to ClinicalTrials.gov. Warning message:
"The study <study title> Primary Completion date is more than 12 months in the past and results have not been submitted to ClinicalTrials.gov. The responsible party should submit results to Clinicaltrials.gov."
Project level and study level clinical trial codes are discrepant Warning messages:
When project-level CT code is No:
"One or more study records is listed as a clinical trial; however, this grant has a clinical trial indicator of No. Please contact your NIH Program Officer."
When project-level CT code is Yes:
"None of the study records are listed as clinical trials; however, the grant has a clinical trial indicator of Yes. Please contact your NIH Program Officer.”
Sec 6. Study Primary Completion Date is missing on the form Error Message:
Study Primary Completion Date is missing for Study<Study Title>. Please click on the Human Subjects link in G.4 to update the Study Primary Completion Date in section 6 of the Human Subjects and Clinical Trials Information form.
Sec 6. Study Final Completion Date is missing on the form Error Message:
Study Final Completion Date is missing for Study<Study Title>. Please click on the Human Subjects link in G.4 to update the Study Final Completion Date in section 6 of the Human Subjects and Clinical Trials Information form.
Sec 6. Completion of primary endpoint date analyses is missing Error Message:
Completion of primary endpoint data analyses date is missing for Study<Study Title>. Please click on the Human Subjects link in G.4 to update the Completion of primary endpoint data analyses date in section 6 of the Human Subjects and Clinical Trials Information form.
Sec 6. Reporting of results in ClinicalTrials.gov is missing. Error Message:
Reporting of results in ClinicalTrials.gov date is missing for Study<Study Title>. Please click on the Human Subjects link in G.4 to update the Reporting of results in ClinicalTrials.gov date in section 6 of the Human Subjects and Clinical Trials Information Form.
Sec 6. Is this an applicable clinical trial under FDAAA answer is missing. Error Message:
Is this an applicable clinical trial under FDAAA answer is missing for Study<Study Title>. Please click on the Human Subjects link in G.4 to update Is this an applicable clinical trial under FDAAA in section 6 of the Human Subjects and Clinical Trials Information Form.
Completion of primary endpoint data analyses date is more than 12 months after the Primary Completion Date Warning Message:
Completion of Primary endpoint data analyses date cannot be later than 12 months from Primary Completion Date for Study <Study Title>. Please click on the Human Subjects link in G.4 to update the Primary endpoint data analyses date in section 6 of the Human Subjects and Clinical Trials Information Form.
Reporting results in Clinicaltrials.gov date is more than 12 months after the Primary Completion Date Warning Message:
Reporting of results in Clinicaltrials.gov date cannot be later than 12 months from primary completion date for Study<Study Title>. Please click on the Human Subjects link in G.4 to update the Reporting of results in ClinicalTrials.gov in section 6 of the Human Subjects and Clinical Trials Information Form.
Jan 22, 2019
eRA Information: New 2-Step Submission Process for RPPRs with Inclusion Enrollment Data
With the launch of the new Human Subjects System (HSS), there is now a new two-step submission process for any Research Performance Progress Reports (RPPR) reporting inclusion enrollment updates. When investigators are completing their RPPR inclusion enrollment updates, the system will now automatically open a separate screen for enrollment data entry. This requires separate submission steps for the enrollment data file and the full RPPR file. The NIH RPPR Instruction Guide has not yet been updated regarding this new process. In the meantime, please see below for additional details and instructions for Signing Officials and Principal Investigators.- Who’s Affected? All principal investigators submitting RPPR files with 2018 inclusion enrollment updates submitted since the June 9, 2018 launch of the Human Subjects System (HSS).
- What’s Changed? There is now a two-step submission process for inclusion enrollment data updates made during the RPPR process – one submission via HSS for the inclusion data and a separate submission for the full RPPR file.
- Investigators will enter their inclusion enrollment updates via HSS in the Human Subjects and Clinical Trial (HSCT) form. When completed, they need to update the HSS file status to “Ready for Submission” and route to their Signing Official (SO) for submission prior to the submission of their full RPPR file.
- Once the SO then submits the updated HSCT form, the RPPR can be completed and submitted as usual. (See below for detailed instructions)
Note: If the SO does not submit the HSS HSCT updates prior to the RPPR submission, the RPPR file will not reflect the updates made to the inclusion enrollment data. Investigators will receive a warning when trying to submit their RPPR (see screenshot below) but this does not prevent them from submitting.Figure 1: Warning Message to Remind Signing Official to Submit HSS Enrollment DataInstructions for Principal Investigators for submitting updated human subjects and enrollment date with the RPPR.- While in the RPPR Menu screen, click the Edit button in the bottom left-hand corner of the screen.
- If inclusion enrollment updates are needed, first save any RPPR data entered thus far, then click the G Special Reporting Req tab.
- Under G.4.b Inclusion Enrollment Data, click the Human Subjects link – this will open a window in HSS to make any edits in the Human Subjects and Clinical Trials (HSCT) form.
- In the Application Information Screen, click on the HSCT Post Submission tab
- In the Study Records screen, click on the View button to see a particular study
Figure 2: Accessing a specific study record in HSS- To make changes, click the Edit button at the top of the screen, just above the SECTION 1 header.
- Scroll down to the bottom of SECTION 2 to find the Inclusion Enrollment Report(s) to be updated.
- Enter the enrollment figures and the system will automatically total the rows and columns. Note: the “Unknown” gender/race/ethnicity categories are not available for the Planned Enrollment tables.
- After updates are made, click Save and Release Lock and the Submission Status (in the Application Information Screen) will change to Work in Progress.
- Back in the Application Information screen, click on the Summary tab near the top of the screen.
- Next click the UPDATE SUBMISSION STATUS button in the Actions pane on the left-hand side of the screen and in the Select the new statusdropdown menu, select Ready for Submission.
- Add a comment and click “add comment,” or click on the blue text stating “continue without adding a comment” to proceed.
- The system will send an email to the Signing Official (SO) to notify them that the updates are ready for submission. The SO must log into eRA Commons and click the Submit Application button to allow the changes to be included in the RPPR and transmitted to NIH.
- Complete the rest of the RPPR updates then save and submit as usual.
Should the RPPR be submitted before the HSCT form, please go to the online help for the Human Subject System (HSS) and refer to the instructions on How to Change the Application Status and Resubmit.If you have additional systems-related issues contact eRA Service Desk.
Jan 07, 2019
eRA Information: Detailed Status Information Unavailable from 9:00 to 10:00 p.m. January 8
Due to upgrades being deployed to the eRA Commons, documents found on the detailed Status Information screen (see Figure 1) will be unavailable tomorrow night, Tuesday, January 8, 2018 from 9:00 p.m. to 10:00 p.m. EST.
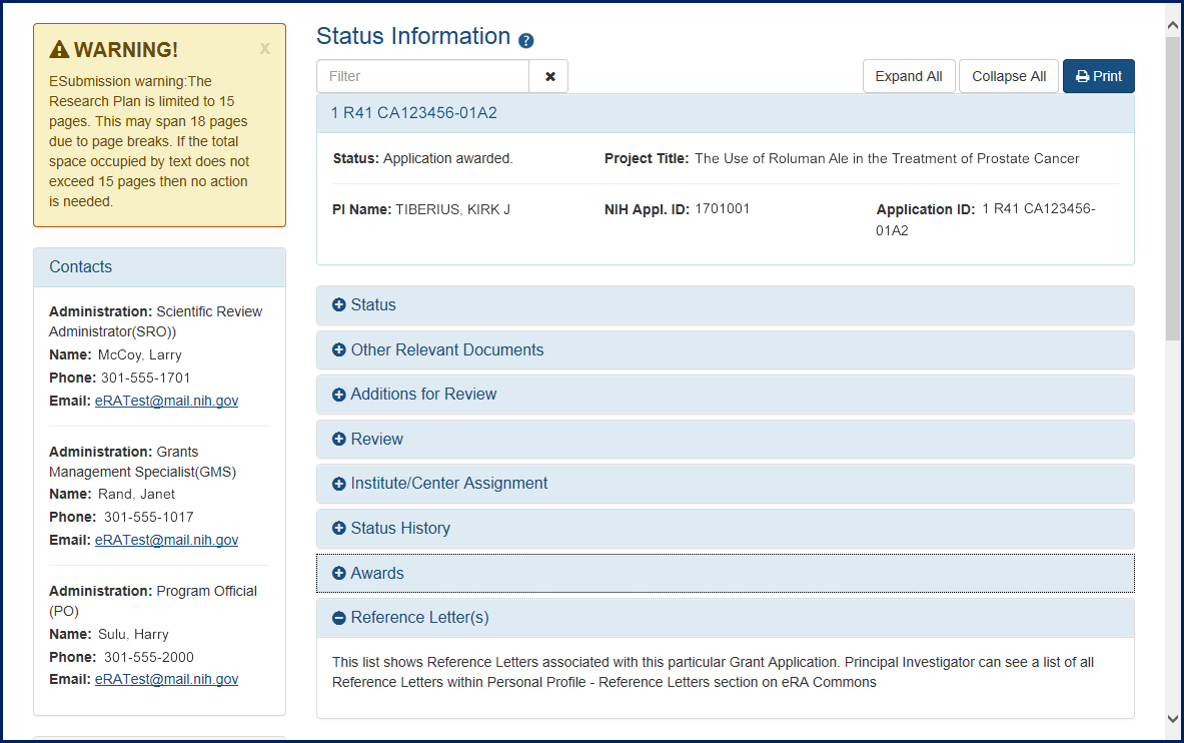
Figure 1: Detailed Status Information Screen
During this time, users will be unable to access grant related documents found on the Status Information screen. If you feel this issue may impinge on your ability to submit an application in a timely manner, please refer to the Dealing with System Issues web page for guidance.
Dec 19, 2018
eRA Information: A Release Starting at 5 PM Tomorrow, Thursday, December 20, Will Temporarily Disrupt Person Search in xTrain
An eRA release planned for tomorrow, Thursday, December 20, will result in disruption of the Person Search feature in xTrain from 5 p.m. to 6 p.m. While all attempts are being made to minimize this disruption we wanted to make you aware that users will not be able to complete a search for a person in xTrain during the specified time.
Oct 18, 2018
New Features In eRA Commons Release Tonight; Connectivity May Be Affected During The Release
eRA is performing software updates to eRA Commons tonight, Thursday, October 18. The release may result in the temporary disruption of connectivity to the module. While all attempts are being made to minimize any lapses in connectivity, we wanted to make you aware that between 8 p.m. EST and 10 p.m. EST, users may not be able to access eRA Commons or may find their connection has been lost. We are sorry for any inconvenience.
New Features & Updates
- Personal Profile on the Go — A mobile version of the Personal Profile screen will be available after the release, giving eRA Commons users access on the go. In addition, security has been enhanced for the entire Personal Profile.
- No Zero for Person Months — A zero can no longer be entered for person months as level of effort in Section D.1 -Participants of the Research Performance Progress Report (RPPR). The zero reflected a time when only whole numbers were allowed, and users were asked to enter a zero if a person month effort for a participant was 0.4 or less. Since decimals are now allowed, the zero is no longer needed.
- Security Enhanced — Security for eRA Commons has been enhanced to keep up with the latest technological advancements.
Oct 16, 2018
PI or PI Delegate Can View No Cost Extension Request Following October 16 Release
eRA performed software updates that modified user access to view the No Cost Extension in eRA Commons today, Tuesday, October 16. There is no action that users need to take and there was no system downtime.
New Update
A principal investigator (PI) or another Commons user to whom a PI has delegated the privilege (PI Delegate) will now be able to view the pdf of the No Cost Extension request submitted to the granting agency. The document will be available on the Status Information screen in eRA Commons under the Other Relevant Documents section. Prior to this release, only the signing official (SO) could view the submitted request in the system.
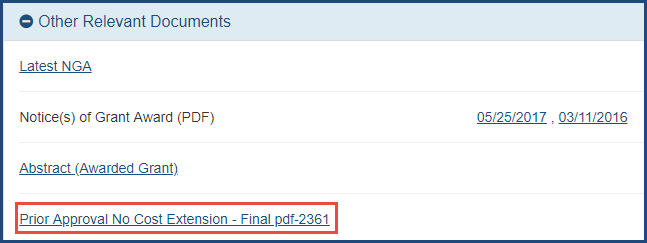
Figure 1: Status Information screen displaying link to submitted No Cost Extension request under the Other Relevant Documents section
As you are aware, if a grant is not eligible for an automatic No Cost Extension, an SO can request prior approval from NIH for a No Cost Extension when the grant meets certain conditions (see Requesting a No Cost Extension in the eRA Commons online help). Note that as before, only the SO can initiate, edit and submit the No Cost Extension.
Sep 25, 2018
eRA Update: Users with ASST Role Can Access HSS Data Only After Application is Initiated by PI or SO
Our September 12 release allowed the contact Principal Investigator (PI) to delegate to a user with the ASST (Assistant) or AO (Administrative Officer) role the ability to work on a Progress Report, including HSS records. However, it has come our attention that users with the ASST role are unable to initiate access to HSS data from the Human Subjects link in Section G.4.b Inclusion Enrollment Data of the RPPR. In the meanwhile, ASST users can access HSS records once the application has been initiated by the PD/PI or SO.
This issue will be resolved in the October 10 release. We are sorry for any inconvenience. Original message sent September 13, 2018, at 10:30 a.m. ET.
Sep 13, 2018
eRA Information: Focused Notifications, Delegation Now Available in HSS
As a result of a software release for the Human Subjects System (HSS) and ASSIST on Wednesday, September 12, the following features and updates have been added.
Human Subject System (HSS)
Focused Notifications
Responding to feedback from the user community, focused notifications will be sent when a user changes the status of updates to human subjects, or clinical trial information to ‘ready to submit’ in HSS:
- Only the Authorized Organizational Representative (AOR) listed on the original submission of the competitive application (type 1 and 2) or change of institution (type 7) will receive these email notifications.
- For HSS changes made on non-competitive years (type 5), the SO associated with the Research Performance Progress Report (RPPR) will be notified.
- If we are unable to identify an email address using either of these choices, notifications will be sent to the institutional contact listed in the Institutional Profile in eRA Commons.
HSS Delegation
Originally, only a Signing Official (SO) could submit HSS records to NIH. With this release of HSS, both the Progress Report delegation and the Submit delegation will be extended to HSS records.
- Progress Report Delegation
- Permits the contact Principal Investigator (PI) to delegate to a user with either the ASST (Assistant) or AO (Administrative Officer) role to work on a Progress Report, including HSS records.
- The SO, Account Administrator (AA), or AO can assign the Progress Report delegation to another PI named on the award, permitting them to work on a Progress Report, including HSS records.
- Submit Delegation
- The Submit delegation can be assigned to the contact PI by the SO, permitting the PI to submit all progress reports for Streamlined Non-competing Award Process (SNAP) awards. The PI is then listed as the Signing Official for that report. This functionality now includes the submission of HSS records.
ASSIST
Applicants can now view budget forms in ASSIST when an application is in a ‘Ready for Submission’ status.
Sep 07, 2018
eRA Update: Plan for Focused Notifications to Designated Signing Official in the Human Subjects System
We wanted to let you know that based on your feedback, we are in the process of fine-tuning the logic for email notifications sent to Signing Officials (SO) when actions are taken in the new Human Subjects System (HSS). Following a release on Wednesday, September 12, we will send a focused notification to the designated SO when a user changes the status of updates to human subjects, or clinical trial information to ‘ready to submit’ in HSS:
- Only the Authorized Organizational Representative (AOR) listed on the original submission of the competitive application (type 1 and 2) or change of institution (type 7) will receive these email notifications.
- For HSS changes made on non-competitive years (type 5), the SO associated with the Research Performance Progress Report (RPPR) will be notified.
- If we are unable to identify an email address using either of these choices, notifications will be sent to the institutional contact listed in the Institutional Profile in eRA Commons.
We are sorry for any inconvenience. We appreciate the constructive feedback we have received on this issue, which helps us to continue making eRA systems work most effectively for you.
Sep 05, 2018
eRA Update: Issue with the Modular Budget Form in ASSIST Has Been Resolved
The inability for users to add a second budget period in the modular budget form in ASSIST was resolved with yesterday’s software release. If any issues persist, please contact the eRA Service Desk at http://grants.nih.gov/support/. (Original message sent Thursday, August 30, 2018 at 2:51 p.m.)
Please Confirm Your Eligibility Status on the Institutional Profile
There are three types, each of which has specific registration requirements:
- Your Organization is eligible to apply for NIH Grants/Contracts.
- Your Organization is eligible to apply for Non-NIH Grants/Contracts.
- Your Organization is eligible to apply for NIH other transaction authority (OTA) opportunities.
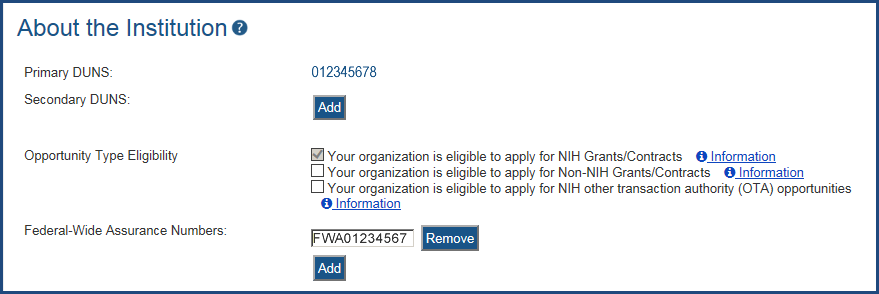
Figure 1: Opportunity Type Eligibility Checkboxes in the Institutional Profile
As part of that process, we added system checks in August 2018 to confirm the eligibility status of the applicant organization for the selected opportunity type(s).
These boxes are checked during eRA Commons registration. Once registration is complete, this information is ported over to the Institutional Profile.
Small business applicants, please review your institutional profile to ensure that you have selected the appropriate opportunity type(s). When an application is submitted to NIH, a system check will be run to verify that the eligibility status of the applicant organization matches the submitted application type (NIH, Non-NIH, or OTA).
For NIH and Non-NIH Grant opportunities, applicant organizations must have a valid DUNS (Dun and Bradstreet Number) at the time they register with eRA Commons.
For OTA opportunities, applicant organizations are not required to have a DUNS number at the time of eRA Commons registration but need to have one before award.
NOTE: Only a person with the signing official role can update the Opportunity Type Eligibility checkboxes.
To review the Institution Profile for these settings:
- Log into eRA Commons
- Click on the Institution Profile tab
- Select the Institution Basic Information sub-tab
- Expand the About the Institution tile
Under the information about DUNS, you will find the Opportunity Type Eligibility section. If this information needs to be updated, click the Edit button on the right side and make the desired changes. Click the Save Changes button at the top of the screen when done.
NOTE: Changing the status of a box does not automatically make you eligible for a specific type of opportunity. It is your confirmation that you have completed all the necessary registrations and eligibility requirements as outlined in the NIH Grants Policy Statement (e.g., Section 2.5.3 determining applicant Organization Eligibility) along with the NIH SF424 (R&R) Application Instructions (e.g., Section 2.2.2 eRA Commons Registration).
See NOT-OD-16-057 for more information.
Aug 30, 2018
eRA Alert: ASSIST Users Currently Experiencing An Issue with the Modular Budget Form
Users have reported an issue with the Modular Budget form in ASSIST (Application Submission System & Interface for Submission Tracking). When users go to add a second budget period and click the SAVE, RELEASE, or CANCEL buttons, they are receiving an error message that the action cannot be completed and that they should contact the eRA Service Desk.
We plan to fix the issue on Tuesday, September 4, 2018. If you feel this issue may impinge on your ability to submit an application in a timely manner, please refer to the Dealing with System Issues web page for guidance. We are sorry for any inconvenience this may cause.
Aug 15, 2018
eRA Update: Plan to Suspend Notifications to Signing Official in the Human Subjects System
As part of the recent release of the Human Subjects System (HSS) functionality, eRA provided an email notification to Signing Officials (SOs) when the PD/PI changed the submission status for a post-submission study record request to ‘ready for submission.’
The idea, based on requests made by the community, was to alert the SO that the updated study was ready to be submitted, rather than having the PI email the SO. The notification went to all listed SOs for an institution. However, after numerous messages voicing concern over the number of notifications all SOs would be receiving, we are planning to suspend notifications effective Friday, August 17, 2018. We will reexamine this process and develop a more efficient method to communicate to SOs when a post-submission request has been set to ‘ready for submission.’
We are sorry for the inconvenience and appreciate the constructive feedback so that we may continue to make eRA systems work most effectively for you.
(Original message sent Tuesday, August 7, 2018 at 3:51 p.m.)
NIH eRA Items of Interest — August 2018
Haaappy Anniversary!
A Continuation of Improvement: IMS to HSS
It is ironic that as I write this, I am celebrating four amazing years of marriage to my wife. And every year on this day I start singing the Flintstones Anniversary song to the tune of the William Tell Overture…
Happy Anniversary
Happy Anniversary
Happy Anniversary Haaappy Anniversary
Happy she and happy heThey're both as happy as can be
Celebrating merrily their happy anniversary…I will never know why that episode has always stuck with me. But I am not the only one who will be celebrating an anniversary. You will be too! And you will know it because we are going to send you an email to honor the occasion. As part of an initiative to ensure your account is up to date, on the yearly anniversary of the creation of your account, you will receive a system generated email asking you to log into eRA Commons, click on the Personal Profile tab, and update any old or errant information. The message will read:
Subject: Annual Validation of Your Personal InformationDear eRA Commons User,This notification is a request for you to validate your personal information within Commons. Please login to Commons and select the Personal Profile tab. Please update any incorrect information.For any further questions about this email, call the eRA Service Desk at 1-866-504-9552 or refer to http://grants.nih.gov/support for additional methods of contact. Please access Commons athttp://public.era.nih.gov/commons/.For more information please visit http://era.nih.gov/The email will use the address listed under the Name and ID section for account-related communications, on your Personal Profile screen. Please ensure all contact information is current, employment information is up to date, and education information is accurate. If you are a reviewer, it also important to make sure Reviewer Communications and Reviewer Payments information is also correct. So Yabba Dabba Dooo! Happy Anniversary!!
From Golden Gates to Nuggets of Knowledge
The NIH Regional Seminar on Program Funding and Grants Administration returns to the West Coast this fall. And as always, you will have the opportunity to collect wonderful nuggets of knowledge (Get it? Gold…nuggets).
If you are new to working with the NIH grants process and want to learn more, while having the opportunity to hear from more than 60 NIH & HHS experts...then this is the opportunity you’ve been waiting for! Optional pre-seminar workshops include detailed information on topics like human research protection, application preparation, post-application submission, intellectual property, iEdison, and more! During the 2-day seminar, you’ll find an array of sessions designed for administrators, researchers, grant writers, etc. In addition, get more personalized guidance during the 1:1 Meet the Expert chats available between attendees and presenters. If that’s not enough...check out these trivia nuggets about the City by the Bay:Here are just local nuggets of San Francisco trivia:- The city was originally named Yerba Buena (Good Herb)
- It has the 2nd largest Chinatown outside of Asia
- There are 50 named hills
- It’s the home of Alcatraz, also known as “The Rock.”
- There are 3500 restaurants available in the 7 by 7 mile city limits
- Bendy straws were invented in 1937 by Joseph Friedman
- In 1927, the electronic television was first demonstrated by its inventor, Philo Farnsworth (who went on to lead research in nuclear fusion.)
Registration is open now! These seminars traditionally reach capacity before the event (and so do the hotel rooms). General registration rates are in effect until September 14, so register soon.When: October 17-19, 2018October 17 – Optional WorkshopsOctober 18 & 19 – 2-day SeminarWhere: Hilton San Francisco Union Square
Aug 13, 2018
Workaround for Viewing Budget Forms in ASSIST When Application Status is Ready for Submission
The August 8 ASSIST release introduced an issue with viewing budget forms in ASSIST when an application is in a ‘Ready for Submission’ status. We will be releasing a fix for this issue on Wednesday, September 12, 2018. This issue does not impact the ability to submit applications.
Symptom:
Some ASSIST users who try to access budget form tabs (RR Budget, Modular Budget, Training Budget) while an application is in a “Ready for Submission” status are encountering the following error message:

Figure 1: Screenshot of message users are getting when they try and view budget forms in ASSIST, when the application is in a ‘Ready for Submission’ status
Workaround:
The forms are viewable when the application is in a ‘Work in Progress’ status. The form is also viewable in the PDF that is generated using the ASSIST Preview Application action. We are sorry for the inconvenience.
Aug 07, 2018
New Features for HSS To Be Released August 8
A new release of the Human Subjects System (HSS) is scheduled for August 8, 2018. The new release will include the following features and functionality:
- Signing officials (SOs) will receive an email when the PD/PI changes the submission status for a post-submission request to ‘ready for submission.’
- A validation has been added that will prevent the user from selecting the same primary enrollment country more than once on the Inclusion Enrollment Report.
- Individuals listed as Multiple Principal Investigators (MPIs) on the original submission will have access to the information in HSS.
- A button to ‘restore previous version’ has been added to the study record form. Selecting this button will void all data entered in the current session and re-initiate the session with the last version of the record submitted to HSS.
- Email notifications regarding access to an application will no longer be sent to the contact PD/PI when program officials and grants management specialists edit a study record for the first time.
For more information on HSS, please see Guide Notice NOT-OD-18-179. You can also reference our HSS Overview and HSS Training pages (for a crosswalk, an infographic and more) and the May Items of Interest article.
Changes to ASSIST Login and Logout with August 8 Release
An ASSIST release scheduled for tomorrow, Wednesday, August 8, will result in some changes to how users log in and log out of ASSIST.
Logging Into ASSIST
If you have bookmarked the login page for ASSIST, you will need to update the link in the bookmark. The URL typically appears as https://public.era.nih.gov/commons. However starting Thursday morning, this URL will no longer work.
If you wish to bookmark the ASSIST login page, you will want to use https://public.era.nih.gov/assist.
Logging Out of ASSIST
A temporary issue has been identified when logging out of ASSIST. Using the ASSIST logout button results in no action happening. To log out of your ASSIST account, you will need to switch over to eRA Commons by clicking on the eRA Commons link at the top right of each page.
And from the Commons interface, click the Logout link, also in the upper right corner (see screenshot below).
We will let you know when the logout issue with ASSIST has been resolved.
Jun 26, 2018
eRA Information: Ignore Manage Access Messages for Post Submission Actions on Human Subject Study Records
The new Human Subjects System (HSS) leverages much of the functionality of ASSIST, including sharing screens and notification processes. We have been made aware that an automated message associated with Manage Access is incorrectly being sent to Principal Investigators (PIs). When NIH staff initiate an update to a human subject study record for the first time, the contact PI is receiving a message informing them that they have edit and view access to the identified application.
The message reads:
Dear <PI NAME>:
You have been given access to the application <APPLICATION ID #> <INSTITUTION NAME> by <NIH STAFF NAME>. To access this application, navigate to the Web page https://public.era.nih.gov/assist and login using your existing eRA username. If you have more than one eRA account, please login with the account you want to use to access this application.
You have been given the following access levels:
- Edit Application All
- View Commons Details
If you have any questions about this email, please contact <NIH STAFF NAME> at <NIH STAFF EMAIL>, who initiated this action. Alternatively, you may contact the eRA Help Desk at 1-866-504-9552 or visit the Grant Support Portal
This issue will be corrected in an upcoming release. In the meantime, any managed access messages that the PI may receive can be ignored. We are sorry for any inconvenience this may cause.
Jun 07, 2018
eRA Information: Human Subjects System Launches June 9; Submit Any Enrollment Updates in IMS by June 8
This is to remind you that on Saturday, June 9, 2018, eRA is launching the Human Subjects System (HSS). This system will replace the Inclusion Management System (IMS), which is being retired on the same day. HSS is a shared system that will enable grant applicants and recipients to electronically report and update their data on human subjects and clinical trials to NIH; and NIH agency staff to monitor and manage the data. eRA plans to migrate enrollment records currently in IMS to HSS before the launch. However, incomplete records will not be migrated. It is important to submit any updates to enrollment records to IMS by June 8, 2018 so that you will not have to re-enter any data. All updates must be submitted to NIH by the signing official no later than June 8, 2018 in order to migrate from IMS to HSS.
Note that if an RPPR is in progress on or before June 8, inclusion data cannot be submitted separately in IMS on or before June 8.
- In this situation, the entire RPPR must be submitted on or before June 8, or
- If the RPPR is submitted on or after June 9, any study data entered on or before June 8 in IMS must be reentered in HSS
If inclusion data has not been completed in IMS on or before June 8:
- PI will need to reenter the inclusion data in HSS on or after June 9.
For more information on this transition, please see Guide Notice NOT-OD-18-179. You can also reference our HSS Overview and HSS Training pages (for a crosswalk, an infographic and more) and the May Items of Interest article. A growing number of HSS tutorial videos can be found in the Human Subjects section of the eRA Videos Tutorials page.
May 24, 2018
eRA Information: Make Sure to Update RPPR and Inclusion Data by June 8, 2018, Before IMS Retirement on June 9
Yesterday morning, we sent out an Items of Interest article that announced the introduction of the Human Subjects System (HSS) and the retirement of the Inclusion Management System on June 9, 2018.We would like to clarify information on Inclusion data and the Research Performance Progress Report (RPPR) as it relates to the IMS retirement scheduled for June 9. Note that if an RPPR is in progress on or before June 8, inclusion data cannot be submitted separately in IMS on or before June 8.
- In this situation, the entire RPPR must be submitted on or before June 8, or
- If the RPPR is submitted on or after June 9, any study data entered on or before June 8 in IMS must be reentered in HSS
If inclusion data has not been completed in IMS on or before June 8:
- PI will need to reenter the inclusion data in HSS on or after June 9.
(Original message sent Wednesday May 23, 2018 at 8:24 a.m.)
May 23, 2018
NIH eRA Items of Interest — May 2018
A Continuation of Improvement: IMS to HSS
 When I was six, my father took me to the grocery store to pick up a few items. Now, I don’t know why, but I stole a pack of gum. And being the master criminal mind that I was, I hid my loot in a small bowl high up on a shelf in the kitchen when we got back home. Probably not my best idea since my father was a State Trooper and over six feet tall. Yes, I was busted (I couldn’t see the gum in the bowl, so I figured he couldn’t either. Who knew?). From that point on, I vowed to myself that I would improve!But far, far better than my early petty crime days, is the new Human Subject System (HSS). HSS is scheduled to go live on June 9, 2018 and will be replacing the Inclusion Management System (IMS). HSS is a shared system that enables grant recipients to electronically report and update their data on human subjects and clinical trials to NIH. The system will also allow NIH agency staff to monitor and manage the data.As of June 9, Principal Investigators and Signing Officials will no longer see “Inclusion” links as an action option, but instead will see “Human Subjects” links on both the Status Search screens and in section G.4.b of the Research Performance Progress Report (RPPR). The RPPR Human Subjects link will only be displayed if the award involves human subjects.On June 9th, IMS will be taken offline and all IMS data submitted to NIH by June 8, 2018 will be migrated to the new system. Now this is important… Updates to the enrollment records in IMS must be submitted no later than Friday, June 8. Updates not submitted by June 8 will not be available in HSS and will need to be re-entered into HSS. Incomplete records, those records not submitted by a Signing Official to NIH by June 8, will not be migrated.For more information on this transition, please see Guide Notice NOT-OD-18-179. You can also reference our HSS Overview and HSS Training pages. A growing number of HSS tutorial videos can be found in the Human Subjects section of the eRA Videos Tutorials page.And to bring closure… Upon the discovery of my loot, I endured an intense and arduous interrogation by my father under which I finally cracked (he looked at me sternly and asked where the gum came from). Back to the store we went where I had to confess my crime, pay for the gum and then be escorted out by a Massachusetts State Trooper in full dress. I always have wondered what the cashier thought about that scene… “Dang, the Staties are really getting tough on crime!”? Oh, and my improvement… I never stole again, nor did I ever hide anything in the kitchen again. Lessons learned.
When I was six, my father took me to the grocery store to pick up a few items. Now, I don’t know why, but I stole a pack of gum. And being the master criminal mind that I was, I hid my loot in a small bowl high up on a shelf in the kitchen when we got back home. Probably not my best idea since my father was a State Trooper and over six feet tall. Yes, I was busted (I couldn’t see the gum in the bowl, so I figured he couldn’t either. Who knew?). From that point on, I vowed to myself that I would improve!But far, far better than my early petty crime days, is the new Human Subject System (HSS). HSS is scheduled to go live on June 9, 2018 and will be replacing the Inclusion Management System (IMS). HSS is a shared system that enables grant recipients to electronically report and update their data on human subjects and clinical trials to NIH. The system will also allow NIH agency staff to monitor and manage the data.As of June 9, Principal Investigators and Signing Officials will no longer see “Inclusion” links as an action option, but instead will see “Human Subjects” links on both the Status Search screens and in section G.4.b of the Research Performance Progress Report (RPPR). The RPPR Human Subjects link will only be displayed if the award involves human subjects.On June 9th, IMS will be taken offline and all IMS data submitted to NIH by June 8, 2018 will be migrated to the new system. Now this is important… Updates to the enrollment records in IMS must be submitted no later than Friday, June 8. Updates not submitted by June 8 will not be available in HSS and will need to be re-entered into HSS. Incomplete records, those records not submitted by a Signing Official to NIH by June 8, will not be migrated.For more information on this transition, please see Guide Notice NOT-OD-18-179. You can also reference our HSS Overview and HSS Training pages. A growing number of HSS tutorial videos can be found in the Human Subjects section of the eRA Videos Tutorials page.And to bring closure… Upon the discovery of my loot, I endured an intense and arduous interrogation by my father under which I finally cracked (he looked at me sternly and asked where the gum came from). Back to the store we went where I had to confess my crime, pay for the gum and then be escorted out by a Massachusetts State Trooper in full dress. I always have wondered what the cashier thought about that scene… “Dang, the Staties are really getting tough on crime!”? Oh, and my improvement… I never stole again, nor did I ever hide anything in the kitchen again. Lessons learned.We Ain’t DUNS Yet!
 Speaking of my father, I need to tell you, as a young man he was incredibly handsome. Like James Dean, Paul Newman, or Marlon Brando handsome. And yet by his mid-30s he was nearly bald. So my entire young life I never knew my Dad with hair.Until one day, totally out of the blue, he walked into my room wearing a toupee. I was shocked and caught off guard. I was also apparently very amused as I laughed for the next several minutes. And to this day, I still feel bad about that. But I wasn’t done yet…And neither is eRA as it continues to work hard to improve all aspects of the NIH grant process. But it is not just NIH grants. As you probably know we also work with several partner agencies, supporting the needs of their business processes and awardees. Because of the varied needs of funding agencies and funding mechanisms, you may notice a change in the Institutional Profile.
Speaking of my father, I need to tell you, as a young man he was incredibly handsome. Like James Dean, Paul Newman, or Marlon Brando handsome. And yet by his mid-30s he was nearly bald. So my entire young life I never knew my Dad with hair.Until one day, totally out of the blue, he walked into my room wearing a toupee. I was shocked and caught off guard. I was also apparently very amused as I laughed for the next several minutes. And to this day, I still feel bad about that. But I wasn’t done yet…And neither is eRA as it continues to work hard to improve all aspects of the NIH grant process. But it is not just NIH grants. As you probably know we also work with several partner agencies, supporting the needs of their business processes and awardees. Because of the varied needs of funding agencies and funding mechanisms, you may notice a change in the Institutional Profile. Under the About the Institution section you will see some new fields regarding your institution’s DUNS number(s). These fields have been added to support Other Transaction Authority (OTA) funding opportunities (OTAs are neither grants nor contracts but a different kind of award) and to summarize your institution’s eligibility to submit to the different types of opportunities. So you see, we are not done either. We are always working to improve and provide the best services we can.And speaking of not being done… after catching my breath and drying my eyes, my father asked what I thought of his new look. And on my honor, with total enthusiasm, my response was, “It looks GREAT!” Really? No, not really.
Under the About the Institution section you will see some new fields regarding your institution’s DUNS number(s). These fields have been added to support Other Transaction Authority (OTA) funding opportunities (OTAs are neither grants nor contracts but a different kind of award) and to summarize your institution’s eligibility to submit to the different types of opportunities. So you see, we are not done either. We are always working to improve and provide the best services we can.And speaking of not being done… after catching my breath and drying my eyes, my father asked what I thought of his new look. And on my honor, with total enthusiasm, my response was, “It looks GREAT!” Really? No, not really.Thank You from My House…
 Just a quick note to thank all the attendess who joined us at the NIH Regional Seminar held in DC this past month. Early feedback indicates it was a very successful 3 days. Here are some fun facts:
Just a quick note to thank all the attendess who joined us at the NIH Regional Seminar held in DC this past month. Early feedback indicates it was a very successful 3 days. Here are some fun facts:- Over 900 of you attended from 47 different US states, and 21 different countries!
- 634 One-to-One appointments made by 283 attendees
- 83 NIH & HHS experts participated in the 3 days
- 14 NIH Institutes & Centers were represented in the areas of grants management, program, review, and policy
- Over 70 NIH and HHS presenters
- 48 different session topics during the 2-Day Seminar
And if that wasn’t cool enough, we are doing it all again in October. But this time we are visiting San Francisco!
Registration is open now!When: October 17-19, 2018October 17 – Optional WorkshopsOctober 18 & 19 – 2-day SeminarWhere: Hilton San Francisco Union Square
Apr 23, 2018
eRA Information: Dealing with Fraudulent Phone Solicitations
We have become aware that some grant applicants and award recipients have been receiving fraudulent calls. The callers may represent themselves as eRA Service Desk team members in an attempt to acquire information or to simply make prank calls.
The fraudulent calls may be convincing because they are making it appear as if the call is coming from the eRA Service Desk. These callers are frequently aggressive and, at times, rude.
Please Note:
- No government grant-making agency will make phone calls or send emails or letters to solicit money or personal banking information from a potential grant recipient.
- There are no processing fees for federal grants.
- Federal grants are not issued for personal use, but are intended for institutions and non-profits to carry out projects with a public purpose.
If you think that someone has fraudulently represented HHS or NIH, please visit https://www.hhs.gov/grants/grants/avoid-grant-scams/ or call the HHS Fraud Hotline at 1-800-447-8477.
Mar 28, 2018
eRA Update: Intermittent Errors and Slowness Issue with eRA Systems Has Been Resolved
eRA Update: Intermittent Errors and Slowness Issue with eRA Systems Has Been Resolved
Wednesday, March 28, 2018
The issues users were experiencing with errors and slowness with eRA Systems appear to have been resolved. If any issues persist, please contact the eRA Service Desk at http://grants.nih.gov/support/
(Original message sent Wednesday, March 28, 2018 at 2:20 P.M.)
eRA Alert: Users Experiencing Intermittent Errors and Slowness with eRA Systems
Wednesday, March 28, 2018
The eRA Service Desk has received reports that users are seeing system errors while working with eRA systems or experiencing slowness. We are working to resolve these issues as quickly as possible. We will provide more information when it is available. We are sorry for any inconvenience this may cause.
eRA Alert: Users Experiencing Intermittent Errors and Slowness with eRA Systems
The eRA Service Desk has received reports that users are seeing system errors while working with eRA systems or experiencing slowness. We are working to resolve these issues as quickly as possible. We will provide more information when it is available. We are sorry for any inconvenience this may cause.
Feb 06, 2018
NIH eRA Items of Interest - February 2018
The Delegations Are Coming, the Delegations Are Coming!
You may recall a quaint article, “Interim RPPR, No Tribble At All,” from February of last year, where I introduced the Interim RPPR (I-RPPR) and how it worked with the Final RPPR (F-RPPR), and Type 2 competing applications.
At the time, policy required that I-RPPR and F-RPPR work in the same manner as the old Final Progress Report (FPR). With the FPR, either the Signing Official (SO) or Principal Investigator (PI) could initiate and submit the report. There were no delegations needed since the FPR was an uploaded PDF, meaning anyone could develop and work on the report. However, the I-RPPR and F-RPPR are submitted online through the Commons in the same format as the annual RPPR. As a result, the most common question we heard at the regional conferences in 2017 was “Can working on I-RPPR and/or F-RPPR be delegated?” We are pleased to announce that it is now possible to delegate working on I-RPPR and F-RPPR to anyone with the Assistant (ASST) role.
As with the old Final Progress Report, only the SO or PI are allowed to submit the I-RPPR and F-RPPR.Also, I am pretty sure Albert Einstein never said the above line. But I do recall this one: “Don’t believe everything you read, even if it is on the internet.” – Albert EinsteinMaintenance On the Move
/ˈmānt(ə)nəns,ˈmān(t)nəns/
noun
The process of maintaining or preserving someone or something, or the state of being maintained.As you know, maintaining anything is important: a car, a hairstyle, the supply of available ice cream. All very important things to maintain! And no less important to maintain are the systems at eRA. So I want to share with you some information about our maintenance schedule.Once a month eRA brings all of its systems offline to conduct routine maintenance. The maintenance is conducted on eRA databases, modules and websites to provide enhanced IT security and increased reliability. Starting in February, we will be moving this process from the customary first weekend of the month to the second weekend of the month. The start time for all scheduled maintenances is 9 p.m. Saturday and concludes at 5 a.m. the next day, per our normal practice.You can find the 2018 Maintenance Calendar on the eRA web site. Updates and additional details about planned maintenance are documented in this calendar as they become available.And I’m off to Baskin-Robbins……Welcome to My House…
May is not that far away! And this year our Regional Seminar is right here in my house… Washington, DC!As always there will be a wealth of information and opportunities for you and your colleagues to learn from NIH faculty and staff! The Seminar starts on May 2 with our Pre-Seminar Workshops, followed on May 3 and 4 with the seminars, discussions and keynote speakers!Need help convincing your boss you should attend? Check out this wonderful video that outlines all the advantages of attending the 2018 NIH Regional Seminar.With the many changes in policies for human subject and clinical trials, new forms, and the discontinuation of PDF form-based applications, can you really afford not to be here? Registertoday before space is sold out!
Jan 05, 2018
eRA Enhancements: New Link for ORCID in Personal Profile
ORCID ID (Open Researcher and Contributor ID), a personal digital identifier that distinguishes every researcher, is used by NIH and Grants.gov to relate publications to grants. In a release yesterday, a new link to access ORCID.org was added to the Personal Profile section of eRA Commons. This will allow principal investigators to create an ORCID ID to link to their Commons account, so that their publications can be linked to their grants.
Figure 1: ORCID ID Link in the User Information section of the Personal Profile
In addition, the Personal Profile screens will be updated on Wednesday, January 10, to better align with best practices in security, user interface design, and industry standards. The navigation will be more user friendly, while the underlying functionality, fields, and requested information will remain the same.
Nov 27, 2017
eRA Enhancements: New Look and Feel Coming Nov. 30 for FCOI Screens in eRA Commons
The Financial Conflict of Interest (FCOI) report in eRA Commons will be enhanced with new screens that are more user friendly to navigate, in a release planned for Thursday, November 30. The changes are designed to better align with best practices in security, user interface design and industry standards. Note that the underlying capabilities, fields and information will remain the same.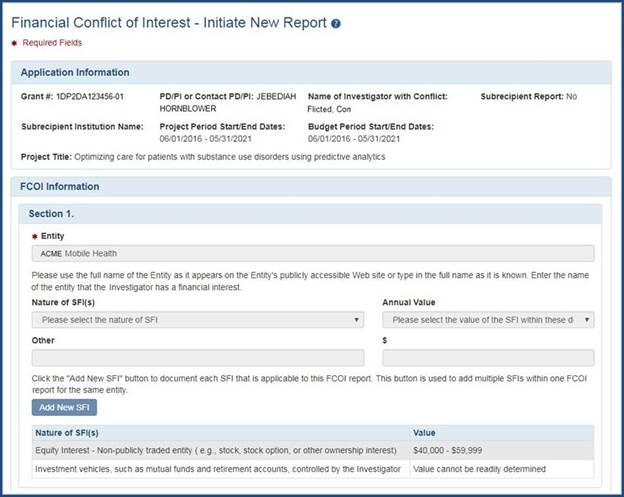 Figure 1: New Initiate New Report screen in FCOI (two-thirds of the screen shown)
Figure 1: New Initiate New Report screen in FCOI (two-thirds of the screen shown)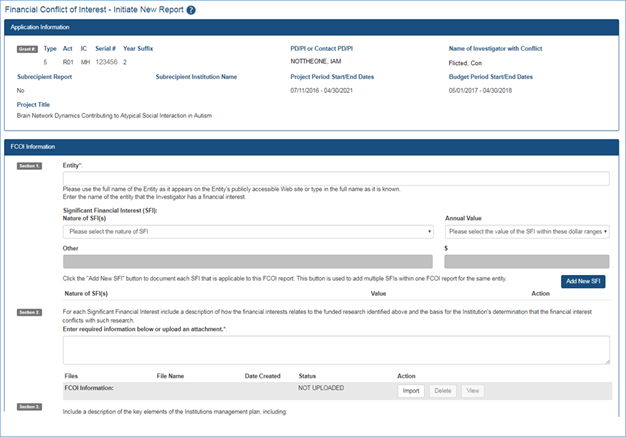 Figure 2: Existing Initiate New Report screen in FCOI (two-thirds of the screen shown)
Figure 2: Existing Initiate New Report screen in FCOI (two-thirds of the screen shown)
Nov 17, 2017
NIH eRA Items of Interest — November 2017
Technical Jargon? Not for Project Outcomes
 At this point it is a pretty well-documented fact that I am a Star Trek fan. But even as a die-hard fan, I sometimes have to wonder what the writers were thinking. Take for example the TNG episode where Capt. Picard needs his artificial heart repaired. In the midst of the operation something goes horribly wrong, and the dialog goes something like this:SURGEON: There's been some capillary reaction here. Let's proceed carefully. We'll need sharper focus on the thoracic polychromatics and verification of myocardial enzyme balance.SURGEON: It's not working. Something's wrong. The metabolation occlusions aren't holding. Damn it! I can't stop the heterocyclic declination. Fuse.SURGEON: Again! We need a biomolecular physiologist in here. This man is dying.WHAT?? Talk about throwing a bunch of big words together. For all I know, it could have meant Picard had a killer case of hiccups. What we need here is some plain language that everyone can understand.And that is exactly what we need in the Project Outcomes section of the Final and Interim Research Performance Progress Reports (F-RPPR and I-RPPR). As you are aware, with NIH’s implementation of the F-RPPR and I-RPPR, a new section, Section I – Outcomes, has been added. The Outcomes section will be made publicly available through the NIH RePORTER website. The objective is to provide the public with easily understood results for NIH-funded biomedical research, thus improving the transparency about the use of federal funds.NIH will only publish project outcomes after they’ve been reviewed and approved by NIH staff. If a project outcome is in biomedical expert-speak, shares proprietary information, or includes Personally Identifiable Information (PII), a grantee will be asked by NIH staff to submit revised project outcomes using the Additional Material functionality for the Final and Interim RPPR (i.e., Final Report Additional Materials (FRAM) for Final RPPR, IRAM for Interim RPPR). Grantees will respond to these requests using an enhanced web form that supports NIH’s ability to make this information public.Submitting a revised outcomes statement is accomplished by using only the Project Outcomes section on the request for Additional Material web form. This text box is NOT for general comments or communication. And revised outcome statements will not be accepted if they are uploaded as an attachment. That functionality is reserved for other types of requested information.
At this point it is a pretty well-documented fact that I am a Star Trek fan. But even as a die-hard fan, I sometimes have to wonder what the writers were thinking. Take for example the TNG episode where Capt. Picard needs his artificial heart repaired. In the midst of the operation something goes horribly wrong, and the dialog goes something like this:SURGEON: There's been some capillary reaction here. Let's proceed carefully. We'll need sharper focus on the thoracic polychromatics and verification of myocardial enzyme balance.SURGEON: It's not working. Something's wrong. The metabolation occlusions aren't holding. Damn it! I can't stop the heterocyclic declination. Fuse.SURGEON: Again! We need a biomolecular physiologist in here. This man is dying.WHAT?? Talk about throwing a bunch of big words together. For all I know, it could have meant Picard had a killer case of hiccups. What we need here is some plain language that everyone can understand.And that is exactly what we need in the Project Outcomes section of the Final and Interim Research Performance Progress Reports (F-RPPR and I-RPPR). As you are aware, with NIH’s implementation of the F-RPPR and I-RPPR, a new section, Section I – Outcomes, has been added. The Outcomes section will be made publicly available through the NIH RePORTER website. The objective is to provide the public with easily understood results for NIH-funded biomedical research, thus improving the transparency about the use of federal funds.NIH will only publish project outcomes after they’ve been reviewed and approved by NIH staff. If a project outcome is in biomedical expert-speak, shares proprietary information, or includes Personally Identifiable Information (PII), a grantee will be asked by NIH staff to submit revised project outcomes using the Additional Material functionality for the Final and Interim RPPR (i.e., Final Report Additional Materials (FRAM) for Final RPPR, IRAM for Interim RPPR). Grantees will respond to these requests using an enhanced web form that supports NIH’s ability to make this information public.Submitting a revised outcomes statement is accomplished by using only the Project Outcomes section on the request for Additional Material web form. This text box is NOT for general comments or communication. And revised outcome statements will not be accepted if they are uploaded as an attachment. That functionality is reserved for other types of requested information.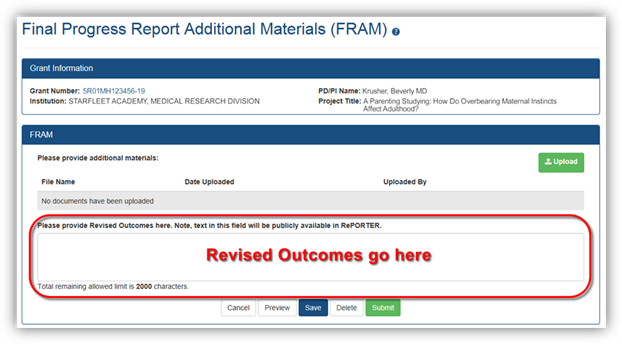
- For the latest Guide Notice on Project Outcomes, please see NOT-OD-18-103.
- For an example of an acceptable Project Outcomes description, please see the Summary of R01HL066004.
- For more information on Interim and Final RPPR, please refer to NOT-OD-17-085 and NOT-OD-17-022.
As for the above dialog, I would suggest:SURGEON: There's been some voltage drop here. Let's proceed carefully. We'll need to closely monitor the voltage / amperage balance.SURGEON: It's not working. Something's wrong. The alligator clips aren't holding. Damn it! I can't stop the power drop!SURGEON: We need an electrician in here. This man is dying.Makes sense to me now.
Nov 06, 2017
eRA Information: Grants.gov Experienced Technical Issues, Now Resolved
It was reported that Grants.gov was experiencing technical issues impacting applicants’ ability to access application packages, submit, check status of a submission and access their website. These issues also impacted some features in ASSIST and NIH’s ability to process applications. It appears that service to Grants.gov has been restored and NIH is now processing all submitted applications. These issues may have been related to a wider Internet issue.
If any issues with Federal systems impact your ability to submit on time to NIH due dates, please follow our guidelines for dealing with system issues. You can take advantage of our online ticketing system and it is acceptable to list multiple impacted applications in the same ticket.
Oct 24, 2017
eRA Information: Ext-UAT and Commons Demo Environment Unavailable From 5 to 6 p.m. Today
The External User Acceptance Test (Ext-UAT) and the Commons Demo environment is scheduled to be offline today, Tuesday, October 24, 2017 from 5 p.m. to 6 p.m. ET for maintenance. We are sorry for any inconvenience this may cause. Please note that this process will not affect the production environment.
Oct 20, 2017
NIH eRA Items of Interest — October 2017
Multiplicity – Fun @ the Movies?

Do you remember this one? Multiplicity is the Michael Keaton movie from 1996. He plays Doug Kinney, a father, husband, and construction worker who has little time for himself. Solution: Get himself cloned! Hilarity ensues (ok, not really) as things go from bad to worse.
While the concept of having multiple “you’s” may seem good, it can be very bad. And this is true in eRA Commons as well. If you have ever attended one of our workshops or seminars, you probably have heard us say that scientists should have only one eRA Commons account that follows them throughout their research career. But sometimes a person gets a second or even a third eRA Commons account created for them.
There are a number of reasons it is important not to have duplicate accounts. NIH needs accurate information to track the careers of NIH funded researchers; it helps in the proper association of committee service for a reviewer to determine Continuous Submission status; and it keeps your grant record history together instead of being split across multiple accounts.
eRA has developed a solution! We sent targeted emails to a sampling of those PIs whom we believe from our records have duplicate accounts and will be sending more in the next two weeks. These PIs will be instructed to go to a new Account Verification screen in Commons. It is located under the Admin tab, which has sub tabs of Accounts, Delegations, and now Account Verification. If a PI has been identified as a person who has multiple accounts, the PI will see the accounts listed on this screen. The PI then will need to confirm that the accounts do belong to him or her, and then select the account that they prefer to be their permanent account. The Data Quality folks will then collapse or converge the data from the multiple accounts to the one the PI specified as preferred.
Those who have current committee or grant involvement are required to select that Commons account as preferred. Here are the instructions for accessing the account verification page and processes to identify preferred and duplicate accounts. Do not act on these until you get an email from eRA communications urging you to do so.
Since multiple Commons accounts create headaches for all involved, there is a lesson in all of this. Whether you are a Signing Official (SO), Account Administrator (AA), or a Principal Investigator (PI), know and understand the process for creating new accounts and affiliating existing accounts when a PI switches institutions. Scientists should never have a new account created for them if one already exists.
Having multiple “me’s” seems like it could be useful, until I realize my clones would all come out just like me. That is to say, just like #4.

If you know anything about computers, let’s face it, you are your parents “tech person.” The call usually goes something like this:
Me: Hi Mom. Everything OK?
Mom: No, I can’t find my email thing.
Me: What thing, Mom?
Mom: You know, that thing I click on to read email. I was reading email, then Betty called, so we talked a bit. After that I decided to play Solitaire. Then I got to thinking about what Betty said about the sale at the mall, so I went online to look up the store hours. And now I can’t find my email thing.
Me: OK, look at the very bottom of the computer screen. Tell me what you see.
Mom: There is blue “E”, no wait, 4 blue “E’s”, then there are, wait, 1, 2, 3, … 6 rainbow looking circles, followed by a little orange cat, or is that a dog, then 5 more “E’s”….
You get the idea. My Mom has a bunch of browser windows open. And now she can’t find anything. Well, eRA Commons that holds and manages all the data about your applications and grants is kind of like my Mom. If you get too many browser windows or browser tabs open, things can get confused, and the result can be bad data.
From a non-technical perspective, when you are working within eRA Commons, your browser makes a secured, encrypted connection with eRA Commons. One connection, one stream of data back and forth, from you to the server. Think of it as a nice two lane highway. But if you open multiple windows, so maybe you can work on various parts of a form at the same time, you are adding additional lanes of traffic on that highway. And like any highway, more traffic creates a greater chance for a crash. Or in our case, creating bad data.
Some advice to save you from frustration later… work in only a single window or tab. We are in the process of updating eRA Commons so that you will be unable to open multiple windows at the same time. This will help to keep your data correct and will let you avoid issues later.
And an unhappy eRA Commons is like an unhappy Mom. If she isn’t happy, nobody is happy!
Oct 18, 2017
eRA Information: eRA Commons, ASSIST Scheduled Downtime For Upcoming Release Thursday, October 19
Scheduled Downtime for the October Release:
eRA Commons and ASSIST will be unavailable beginning at 9 p.m. ET Thursday, October 19 and will return to service by 7 a.m. ET Friday, October 20, 2017.
The downtime will allow for the deployment of updates in eRA’s October system-wide software release.
NOTE: The ability to submit help tickets online will not be available during the downtime listed above.
Sep 19, 2017
eRA Enhancement: Ability for Agency to Request Additional Materials for Interim RPPR via Commons Coming September 20, 2017
A new capability will be added to eRA Commons during a software release on Wednesday, September 20, 2017. There is no anticipated downtime during this release. Awarding agencies will be able to request additional materials for an Interim RPPR from the principal investigator (PI) and signing official (SO) via eRA Commons. In turn, the SO will be able to submit the additional materials via eRA Commons, in a process that is similar to the Final Progress Report Additional Materials (FRAM) process.
The SO and PI will receive an email request from the program official at the awarding agency. They will also see the Interim Progress Report Addition Materials (IRAM) link requesting the information on the Status Results screen, in the Available Actions column. As with the RPPR, a PD/PI (or Contact PI, in the case of multiple PIs) can enter the IRAM. However, only the SO can submit an IRAM to the agency.
For detailed information and screenshots, please see the Latest News section in the eRA Commons online help, following the release.
Aug 23, 2017
eRA Enhancements: New Features for xTRACT Coming August 24, 2017
Several new features will be added to xTRACT in a software release scheduled for Thursday, August 24, 2017. xTRACT is the Extramural Trainee Reporting and Career Tracking system and is accessed via eRA Commons. It allows applicants, grantees and assistants to create research training tables for progress reports and institutional training grant applications.
Features
Upload Feature for Participating Trainee Data
Users will now be able to upload participating trainee information in batches, by providing a file separated by tabs that contain certain information for each trainee. For each row of information in the file, a participating trainee will be either inserted or updated as appropriate. For each trainee listed in the upload file, the following information shall be included:
- The Trainee's Commons User Id
- Trainee Type (Pre-Doc, Post-Doc, or Short-Term)
- In-Training Indicator ("Y" for yes, or "N" for no)
- Start Date (in MM/YYYY format)
- End Date (in MM/YYYY format)
- Research Topic (up to 200 characters)
- Commons ID of first associated Faculty Member
- Commons ID of second associated Faculty Member
NOTE: You may choose to create your file using a spreadsheet application, and then saving the file as a “tab-delimited” text document.
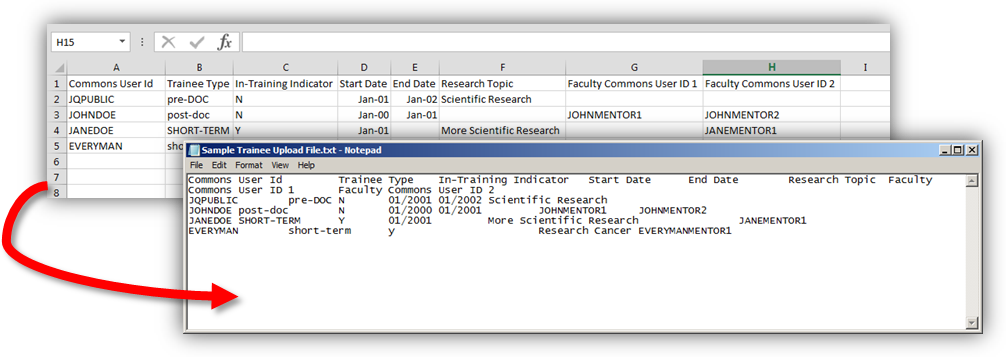
Upload Feature for Participating Student Data
Similar to the participating trainee upload described above, users will also be able to perform a batch upload of participating student information. The information included in the upload file will follow the same format as participating trainees, with one exception: the only allowable values for Student Type are Pre-Doc and Post-Doc (no short-term).
Following the release, please look for details and screenshots in the Online Help for xTRACT (and accessible through the question marks on xTRACT screens).
Aug 15, 2017
eRA Information: Ext-UAT Environment for eSubmission and ASSIST Unavailable Wednesday, August 16, 2017
The External User Acceptance Test (Ext-UAT) environment for eSubmission processes and ASSIST is scheduled to be offline on Wednesday, August 16, 2017 from 8 a.m. to 6 p.m. ET. This is in preparation for implementing new Human Subject and Clinical Trial (HSCT) forms and validations. As you are aware, for Funding Opportunity Announcements with due dates on or after January 25, 2018, all applications proposing human subject studies or clinical trials will be required to use the HSCT form. (See Guide Notice NOT-OD-17-062)
We are sorry for any inconvenience this may cause. Please note that this process will not affect the production environment.
Jul 21, 2017
eRA Update: Users with the Status Delegation Can Now Access the Detailed Status Information Screen
Users with the ASST (Assistant) role, that have been assigned the Status delegation for a Principal Investigator (PI) are now able to see all information on the detailed Status Information screen for that PI. Thank you for your patience.
Original message sent Friday, July 21, 2017 at 12:32 p.m. ET.
eRA Alert: Users with the Status Delegation Unable to Access the Detailed Status Information Screen
The eRA Service Desk has received reports, users with the ASST (Assistant) role, that have been assigned the Status delegation for a Principal Investigator (PI) are currently seeing an error on the detailed Status Information screen for that PI. We are working to actively resolve these issues and will provide more information when it is available.
We are sorry for any inconvenience this may cause.
Jul 18, 2017
eRA Information: Brief Downtime for eRA Website at 8 p.m. on July 19, 2017
The eRA web site (https://era.nih.gov) will be offline Wednesday evening from 8 p.m. to 9 p.m. ET for required maintenance. The maintenance will only affect the website. Production systems such as eRA Commons, IAR and ASSIST will be available and fully functional. You can access eRA Commons and IAR by using this URL: https://public.era.nih.gov/commons
And ASSIST can be accessed at: https://public.era.nih.gov/assist
We are sorry for any inconvenience.
eRA Information: Scheduled Downtime for eRA Commons, ASSIST Starts Thursday, July 20
Scheduled Downtime for the July Release:
eRA Commons and ASSIST will be unavailable beginning at 9 p.m. ET Thursday, July 20 and will return to service by 7 a.m. ET Friday, July 21, 2017. The downtime will allow for the deployment of updates in eRA’s July system-wide software release. NOTE: The ability to submit help tickets online will not be available during the downtime listed above.
Jul 14, 2017
eRA Information: Ext-UAT and Commons Demo Environment Will be Unavailable Tuesday, July 18, 2017
The External User Acceptance Test (Ext-UAT) and the Commons Demo environment is scheduled to be offline Tuesday, July 18, 2017 from 8 a.m. to 6 p.m. ET in preparation for the summer system-wide software release. As part of our standard practice before each quarterly software release, we update the Ext-UAT and Commons Demo environments with the changes that will take place in production.
We are sorry for any inconvenience this may cause. Please note that this process will not affect the production environment.
Jun 09, 2017
Issue with ASSIST Uploads Resolved
We have resolved the issue that affected uploads of some pdf documents to ASSIST. Thank you for your patience.
Jun 07, 2017
Issue with ASSIST Uploads
A technical issue is affecting uploads of the following pdf documents to ASSIST:
- introduction for resubmissions (A1)
- progress report for renewal (type 2) applications.
We are working to quickly resolve the issue and will keep you updated. Applicants affected by this issue should contact the eRA Service Desk (see Dealing With System Issues). We are sorry for any inconvenience.
Prior Approval to Include Carryover Option Effective June 8
The ability to electronically submit a carryover request to NIH will be available through the Prior Approval module in eRA Commons after a release scheduled on Thursday, June 8, 2017 after 5 p.m. Carryover allows leftover, unobligated grantee funds to be carried over from one budget year to another, and may require permission be obtained from the awarding IC.
The Prior Approval module currently supports the electronic submission of the following requests:
- Withdrawal of an application
- $500K or more in direct costs
- Change of PD/PI
- No Cost Extension requiring Prior Approval
It is important to note that these features are optional and currently apply only to NIH awards. You can learn more about these electronic submission options by reviewing the tutorials found at: https://era.nih.gov/era_training/era_videos.cfm#eracommons.
Features
- Carryover Request
Only a Signing Official (SO) will be able to initiate the carryover request.
- When is a grant eligible for a carryover request?
- The grant does not have expanded authority.
- The Project period has not ended or a no-cost extension (NCE) request has been submitted by the institution requesting additional time.
- The grant is not in closeout.
- The grant has not been closed.
- What information will an SO need to provide for a carryover request?
- The carryover request form requires:
- Amount of funds to be carried over
- Explanation of unobligated balance
- Detailed Budget
- Scientific Justification
The high level steps In Prior Approval are as follows:
- A list of eligible grants will be displayed.
- The SO will pick a grant and initiate the request
- If there is an overdue Federal Financial Report for that grant, the system will issue a warning. The SO can choose to ignore the warning and continue. However, the FFR will have to be submitted before the carryover can be approved by the funding agency.
- If the SO wants to initiate a no cost extension request in tandem with the carryover request (and it is within 90 days of the project period end date), a button will allow the SO to do that without searching for the grant again.
Any clarifications and back and forth between the SO and the program official will occur outside of the system. Please note that the subsequent review and approval process remains the same. Following the release, please look for details and screenshots in the eRA Commons Online Help. The video tutorial for the Prior Approval Carryover Request can be found at: https://era.nih.gov/era_training/era_videos.cfm#carryover
Jun 01, 2017
eRA Information: ASSIST Budget Forms Issue Resolved
Late in the afternoon on May 31, it was discovered that a technical issue was preventing ASSIST users from completing the Modular Budget Form and several other R&R Budget forms. This issue is now resolved, and users are able to complete these budget forms. If any issues persist, please contact the eRA Service Desk at http://grants.nih.gov/support/.
May 30, 2017
NIH eRA Items of Interest — May 2017
Carryover and Carry On… My wayward Son
When I was asked to do an Items of Interest on Carryover, my brain immediately thought “carry on,” then quickly added “my wayward son.” Ahh, Kansas, Leftoverture, 1976. It is kind of ironic that the name of that album is Leftoverture. Leftover… carryover. Somehow it is all connected, like 6 Degrees of Kevin Bacon.Carryover is a process in which unobligated funds remaining at the end of the budget period (leftover funds) may be carried forward to a future budget period. In the past, the request to carryover funds from one budget period to another would have been done with the Principal Investigator emailing (with the Signing Official’s sign off) the Grants Management Officer (GMO) to explain why there are funds remaining and to get permission to carry the funds over.Starting in June 2017, there will be an option to submit Carryover requests electronically under the Prior Approval Module. Carryover requests will join Withdrawal of an Application, $500K or More Requests, Change of PD/PI Requests, and No Cost Extension Requests as an optional electronic submission method.Grantees are allowed automatic carryover of funds if they have the expanded authorities to do so. For those awards that do not have expanded authorities, grantees need to submit a carryover request to their respective GMO for prior approval. This can be done electronically via the Prior Approval module in eRA Commons, scheduled for early June. Note that the electronic method is optional.Only a Signing Official can submit the request.A Carryover request will be available in Prior Approval when the grantee has met these two conditions:- Prior year’s FFR (Federal Financial Report) has been submitted.
- The grant is still active, meaning the project period has not ended and a no-cost extension (NCE) request has been submitted by the institution requesting additional time, and the grant has not been closed.
As part of the process, several key pieces of information will be required to complete the request:- Unobligated funds to be carried over
- Explanation of unobligated funds
- Budget Justification
- Scientific Justification
Closely related to the Carryover request is the No-Cost Extension request. If a Carryover request is made within 90 days of the project period end date, the Prior Approval Module will allow you to initiate the No-Cost Extension request at the same time as the Carryover request.So, now (sing it with me people) lay your weary head to rest, don’t you cry no more, because Carryover will let you carry on!Latest Video Releases
 Grab a cold drink, a box of “pup-corn” and enjoy the latest eRA video tutorials. With the Prior Approval module expanding rapidly, we recently added four tutorials demonstrating the functionality of:And coming soon will be the Carryover Request tutorial (see above for information). Now, you may find it odd that the links above don’t take you straight to the videos. They take you to a page that then links to the videos on YouTube. Trust me, I am not intentionally making your life more difficult.The videos are hosted on YouTube. But, since a YouTube URL can only be used once, when these tutorials get updated, the URL will also be updated. By bringing you to our launch page first, we ensure you will get to the latest, most recent versions of the tutorials. So enjoy and happy viewing!
Grab a cold drink, a box of “pup-corn” and enjoy the latest eRA video tutorials. With the Prior Approval module expanding rapidly, we recently added four tutorials demonstrating the functionality of:And coming soon will be the Carryover Request tutorial (see above for information). Now, you may find it odd that the links above don’t take you straight to the videos. They take you to a page that then links to the videos on YouTube. Trust me, I am not intentionally making your life more difficult.The videos are hosted on YouTube. But, since a YouTube URL can only be used once, when these tutorials get updated, the URL will also be updated. By bringing you to our launch page first, we ensure you will get to the latest, most recent versions of the tutorials. So enjoy and happy viewing!From Blue Masks to Blue Crabs
 Last week we completed the first of two NIH Regional Seminars on Program Funding and Grants Administration in New Orleans. With an attendance of approx. 870 participants, 70 NIH & HHS faculty and support staff, and 40 volunteers…it was a very busy and successful three days.
Last week we completed the first of two NIH Regional Seminars on Program Funding and Grants Administration in New Orleans. With an attendance of approx. 870 participants, 70 NIH & HHS faculty and support staff, and 40 volunteers…it was a very busy and successful three days. But if you didn’t have the opportunity to join us in New Orleans, fear not, because we will do it all again in Baltimore. The next NIH Regional Seminar will be October 25 – 27, 2017 at the Renaissance Baltimore Harborplace Hotel, in the historic Inner Harbor. With several hundred people already registered for Baltimore, don’t delay in getting yourself registered because space is limited and these events do fill to capacity. Register today to ensure your chance to learn directly from NIH & HHS experts.
Joe SchumakereRA CommunicationsDivision of Communications and OutreachNIH Office of Extramural ResearchQuestions? If you have a question about this email, please contact the eRA Service Desk at https://grants.nih.gov/support/ (preferred method of contact) or call 1-866-504-9552/301.402.7469.Help us improve our communications; send your suggestions and feedback to eRACommunications@mail.nih.gov or call 301-435-8185.To read other recent articles and messages, please visit our Latest News page at https://www.era.nih.gov/news.We are sorry if you receive duplicates. This notification was sent to multiple distribution lists. To subscribe to or unsubscribe from our listservs, please visit https://era.nih.gov/about_era/get_connected.cfm.
May 25, 2017
eRA Alert: Some Automated Email Notifications Not Being Sent
Due to a technical issue, some email messages that are automatically generated are not being processed. These messages will be queued to be resent once the issue is resolved. We will provide more information when it is available. We are sorry for any inconvenience this may cause.
iEdison Online Help
May 24, 2017
Please Do Not Work on RPPRs between 7 a.m. and 10 a.m. ET on Thursday, May 25
eRA will be deploying a release Thursday morning, May 25, to update the publications section of eRA Commons to accept preprints (See NIH Guide Notice NOT-OD-17-050). While eRA Commons users will not experience any downtime during this release, those who are working on Research Performance Progress Reports (RPPR) may experience errors. Hence, we request users to refrain from working on RPPRs during the 3 hour release window Thursday (7 a.m. to 10 a.m. Eastern Time).
We are sorry for any inconvenience.
May 18, 2017
xTrain’s Statement of Appointment PDF Form Will No Longer Display PII Information
The xTrain module will no longer display certain information in the Statement of Appointment PDF form (PHS 2271), in order to protect Personally Identifiable Information (PII). This change will be effective today, Thursday, May 18, following eRA’s software release. The following information will not be displayed:
- Date of Birth
- Last four digits of Social Security numbers
- Gender
- Race/Ethnicity
- Disability
- Disadvantaged Background
A box has been added next to each of these fields in the PDF of the form, which will be checked if the appointees have completed these fields in their eRA Commons Personal Profile. If they did not provide this information in their profile, these boxes will be left blank.
Apr 27, 2017
eRA Information : Users Experienced Errors When Viewing an RPPR This Morning
The eRA Service Desk has received reports that some eRA Commons users experienced errors when viewing Research Performance Progress Reports (RPPR). As you may know, eRA systems are dependent on the overall NIH infrastructure. We continue to work closely with groups responsible for NIH infrastructure to avoid these issues. If you continue to experience problems, please contact the eRA Service Desk.
We are sorry for any inconvenience.
Apr 25, 2017
eRA Enhancements: New Features for xTRACT Coming April 27, 2017
A new feature and updates to xTRACT will occur in a software release scheduled for Thursday, April 27, 2017. xTRACT is the Extramural Trainee Reporting and Career Tracking system and is accessed via eRA Commons. It allows applicants, grantees and assistants to create research training tables for progress reports and institutional training grant applications.
Feature
- Longer Research Topics are now supported:
When entering the in-training data for a participating student or trainee, the allowable length of the Research Topic has been increased, from 80 to 200 characters.
Updates
- Corrected styling of author names, for citations selected from PubMed
For any publication that is being selected from PubMed and added for a student/trainee, each author name for the citation should now appear in NLM style.
- Corrected Year Awarded, Shown in Table 8 for Non-NIH Subsequent Grants
Occasionally the year awarded would be shown incorrectly for a Non-NIH subsequent grant in table 8. This problem has been corrected. Following the release, please look for details and screenshots in the Online Help for xTRACT (and accessible through the question marks on xTRACT screens).
Apr 20, 2017
eRA Information: Scheduled Downtime for eRA Commons, ASSIST Begins Tonight at 9 p.m.
Scheduled Downtime for the April Release:
eRA Commons and ASSIST will be unavailable beginning at 9 p.m. ET Thursday, April 20 and will return to service by 7 a.m. ET Friday, April 21, 2017. The downtime will allow for the deployment of updates in eRA’s April system-wide software release. NOTE: The ability to submit help tickets online will not be available during the downtime listed above.
Apr 12, 2017
eRA Information: Ext-UAT and Commons Demo Environment Will be Unavailable Friday, April 14, 2017
The External User Acceptance Test (Ext-UAT) and the Commons Demo environment is scheduled to be offline Friday, April 14, 2017 from 8 a.m. to 6 p.m. ET in preparation for the spring system-wide software release. As part of our standard practice before each quarterly software release, we update the Ext-UAT and Commons Demo environments with the changes that will take place in production.
We are sorry for any inconvenience this may cause. Please note that this process will not affect the production environment.
Mar 30, 2017
eRA Enhancements: New Features for eRA Commons Status Information Screen Released Today
New enhancements have been added to the detailed Status Information screen in today’s software release. The detailed Status Information screen, accessible by Signing Officials (SOs) and Principal Investigators (PIs), is the information screen for every successfully submitted grant application and every grant award.Quick New Access to Important DocumentsIn the Other Relevant Documents section, you have quick access to important forms, documents, and reports. Administrative Supplements and Relinquishing Statements that have been submitted can also be accessed here by clicking on the appropriate link.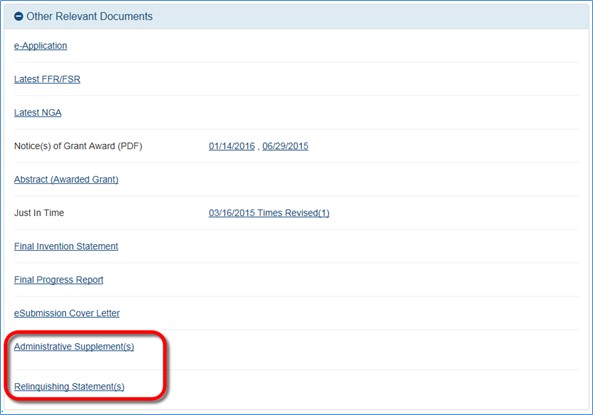
Figure 1: Other Relevant Documents Section Showing Administrative Supplement and Relinquishing Statement links New with this release, these links open new screens that reflect the new look and feel of eRA Commons. Each screen shows an Application ID link, under an Identifying Information header, that opens the submitted documentation. The most recent status of the requests, the date and time the submission was made, and any comments from NIH staff can also be seen on the new screens.
Figure 2: Administrative Supplements Screen Accessed from Status Information
 Please look for details and screenshots in the eRA Commons Online Help.
Please look for details and screenshots in the eRA Commons Online Help.
Mar 24, 2017
eRA Update: Connection Issue to eRA Systems Has Been Resolved
The issue affecting user ability to log in to eRA Commons, ASSIST, IAR, iEdison and related services has been resolved. If any issues persist, please contact the eRA Service Desk at http://grants.nih.gov/support/
(Original message sent Friday, March 24, 2017 at 1:20 p.m. ET)
eRA Alert: Users Experiencing Connection Issues to eRA Systems
The eRA Service Desk has received reports that users of eRA Commons, ASSIST, IAR, iEdison and other related services are unable to log in. The problem has been identified as an NIH wide issue. We will provide more information when it is available. We are sorry for any inconvenience it may cause.
Mar 20, 2017
eRA Enhancements: xTRACT Enhancements Coming March 21, 2017
Several new enhancements will be added to xTRACT in a software release scheduled for Tuesday, March 21, 2017. xTRACT is the Extramural Trainee Reporting and Career Tracking system and is accessed via eRA Commons. It allows applicants, grantees and assistants to create research training tables for progress reports and institutional training grant applications.
Participating Faculty Upload:
The length of each faculty member's research interest is now validated, and an error message is displayed if it exceeds the maximum of 60 characters.
Copy RTD Features:
Some users had occasionally encountered an unexpected error upon attempting to copy a prior RTD. This issue has been corrected.
Correct Mean Values Displayed Properly:
When preparing the data for Predoctoral Applicants/Entrants, the mean values for Total Applicant Pool and Applicants Eligible for Support were sometimes displayed on the Summary tab in reverse order. This has been corrected.
Note that this display issue only affected the values displayed on the screen under the Summary tab -- when viewing these same values in the Preview or Finalized PDF, they have always been presented in the correct order.
Mar 03, 2017
NIH eRA Items of Interest — March 2017
New Orleans: Where the Blues Make You Happy, and the Food Makes You Hungry… for More!
Franklin Delano Roosevelt once said of New Orleans, “New Orleans makes it possible to go to Europe without ever leaving the United States.” And NIH is proud to say we will be in New Orleans from May 3 to May 5, for the first of two* NIH Regional Seminars on Program Funding and Grants Administration in 2017! And what an amazing three days it will be with over 65 NIH and HHS experts on hand just for you. Of course, you will want to check out the agenda for yourself, but here are the highlights: You Hungry… for More!
Wednesday, May 3: Pre-Seminar Workshops
- Full-Day Human Research Protections Workshop
- Half-Day Administrator’s Boot Camp
- Half-Day eRA Workshops (Pre and Post)
- Half-Day Intellectual Property: Understanding Requirements and Recipient Rights & Responsibilities (Beginner)
- Half Day Intellectual Property: An In-Depth Look at iEdison (Intermediate to Advanced)
Thursday, May 4 and Friday May 5: 2 Day Seminar- Keynote speakers
- Topic based presentations organized by track:
- Administrators
- New Investigators
- All Interest
- 1:1 Meet the Expert Sessions (where you get to sit down, face to face with NIH faculty to discuss your needs)
- NIH booths (for more direct access to faculty, technical support, and policy folks)
- Exhibitors
Registration is easy and the seminar hotel is the beautiful New Orleans Marriott right on Canal Street! Early registration ends March 3, 2017, so don’t delay!
So join us for French Creole cuisine, Southern hospitality, European architecture, and American born music, jazz. And you don’t even have to have a passport!
*If these dates or the location don’t work for you, registration for our 2nd seminar in Baltimore, MD (Oct. 25-27) will open this spring.
Joe Schumaker
eRA Communications
Division of Communications and Outreach
NIH Office of Extramural ResearchQuestions? If you have a question about this email, please contact the eRA Service Desk at http://grants.nih.gov/support/ (preferred method of contact) or call 1-866-504-9552/301.402.7469. Help us improve our communications; send your suggestions and feedback to eRACommunications@mail.nih.gov or call 301-435-8185. To read other recent articles and messages, please visit our Latest News page at https://www.era.nih.gov/news.
We are sorry if you receive duplicates. This notification was sent to multiple distribution lists. To subscribe to or unsubscribe from our listservs, please visit http://era.nih.gov/about_era/get_connected.cfm.
Feb 28, 2017
eRA Enhancements: Two More Prior Approval Requests Go Electronic March 2, 2017
The ability to request a No Cost Extension requiring a prior approval and the ability to request a change of PD/PI will be available through the Prior Approval section in eRA Commons after a release scheduled for Thursday, March 2, 2017.
The Prior Approval tab was introduced to eRA Commons in September 2016 to initially provide an electronic option for executing a request for application withdrawal (see Guide Notice NOT-OD-143). In October 2016, the ability to request to submit an unsolicited application with $500K or more in direct costs was added to Prior Approval (see Guide Notice NOT-OD-17-005).
It is important to note that these features currently only apply to NIH awards. They are also an optional method. You should contact your awarding IC to determine the best method for making these requests before initiating either a No Cost Extension or Change of PD/PI.
Features
- Prior Approval Request for No Cost Extension (NCE)
Signing Officials (SOs) will be able to request an NCE electronically through eRA Commons via Prior Approval.
- When is a grant eligible for a NCE through Prior Approval?
- When an NCE under expanded authority has already been used and the grant is within 90 days of the project end date.
- When the grantee is not under expanded authority and the grant is within 90 days of the project end date.
- When the project end date has expired and has not been closed or has not entered unilateral closeout, whichever comes first.
- When is a grant NOT eligible for a NCE through Prior Approval?
- When an NCE under expanded authority has never been requested and the grant is within 90 days of the project end date. In this case,
- the NCE will be processed normally through the Extension link in Status.
- When the grant is closed.
- When the grant is a fellowship grant.
- What information will an SO need to provide?
- The NCE request form includes:
- Request Detail – Here you will be asked such things as the number of months you wish to extend the project end date; the amount of unobligated money still available, etc.
- Three PDF upload fields: Progress Report, Budget Document, Justification Document
- The NCE request form includes:
Contact the awarding IC for details on the content of these documents.
- Prior Approval Request for Change of PD/PI
Signing Officials (SOs) can initiate the request for a Change of Program Director/Principal Investigator (PD/PI) electronically through eRA Commons via Prior Approval.
- The following conditions must be met for a grant to be eligible for a Change of PD/PI Request:
- The grant is awarded, and the Project Period End Date has not passed.
- The grant is not a Fellowship or Career.
- The details for the request require some basic information:
- Who is being replaced, removed or added to the grant?
- What will their level of effort be?
- What is the effective start date for the requested changes?
- Additionally, some files will need to be uploaded as an attachment to the request.
- Biosketch for any new PD/PI
- Other Support for any new PD/PI
- Justification Document
Once the request is submitted, the system creates a PDF of all the submitted information and sends a notification to the SO, the Grants Management Specialist, and Program Officer so they can review the request.
NOTE: Principal Investigators cannot see Change of PD/PI Requests. Please note that the subsequent review and approval process remains the same. Following the release, please look for details and screenshots in the eRA Commons Online Help.
Feb 17, 2017
eRA Information: Grants.gov Downtime Will Impact ASSIST Ext-UAT
The External User Acceptance Test (Ext-UAT) for ASSIST will be unavailable due to maintenance and upgrades at Grants.gov. Because Grants.gov’s UAT environment works with ASSIST UAT, any transactions between the systems will not be available while Grants.gov completes its maintenance and upgrades to its UAT environment.
Starting at 12:01 a.m. ET on Tuesday, February 21, 2017 (after midnight on Monday), the environments will go offline as Grants.gov updates its UAT environment from HTTP to HTTPS per federal mandate (OMB M-15-13, dated June 8, 2015).
Note: The Grants.gov upgrades will not affect the production environments.
Feb 14, 2017
eRA Enhancements: New Features for xTRACT Coming February 16, 2017
Several new features will be added to xTRACT in a software release scheduled for Thursday, February 16, 2017. xTRACT is the Extramural Trainee Reporting and Career Tracking system and is accessed via eRA Commons. It allows applicants, grantees and assistants to create research training tables for progress reports and institutional training grant applications.
Features
- Text styling of names that are in all-caps:
Any names that are in all-caps, which a user is not otherwise able to enter or edit, are now "styled" to provide a more attractive appearance (initial-caps for each word in the name). This includes:
- Person names (faculty, students, trainees, mentors)
- Institution names (prior institutions, employing organizations, grantee organizations)
- Department names (participating departments, employing organizations/departments, grantee organizations/departments)
- Upload feature for participating faculty members on a Research Training Dataset (RTD).
When adding participating faculty to an RTD, users now have the ability to upload a tab-delimited file of faculty members, rather than entering them by hand. For each faculty member, the following details may be provided in the upload:
- Identification of the faculty member via Commons User ID
- Faculty details consisting of rank, research interest, and training roles
- Mentoring record consisting of: Predocs in Training, Predocs Graduated, Predocs Continued in Research or Related Careers, Postdocs in Training, Postdocs Completed Training, Postdocs Continued in Research or Related Careers
Updates
Ability to cite publications with a large number of authors
Publications can now be added for a trainee if the Pubmed citation lists more than 256 authors.
Year Awarded Correctly Shown in Table 8 for Subsequent Grants
The year awarded is now being displayed correctly for all subsequent grants in table 8. Following the release, please look for details and screenshots in the Online Help for xTRACT (and accessible through the question marks on xTRACT screens).
Feb 03, 2017
NIH eRA Items of Interest — February 2017
Interim RPPR, No Tribble At All
 Like those little fuzzy, purring, furry Tribbles that came cascading out of the grain silo onto Captain Kirk, we have one more RPPR to bounce off you!
Like those little fuzzy, purring, furry Tribbles that came cascading out of the grain silo onto Captain Kirk, we have one more RPPR to bounce off you!As you may recall we started the transition to the Research Performance Progress Report (RPPR) back in April of 2012. And now, with NIH having transitioned to the Final RPPR as of January 1, 2017, we have one more transition to make. And that is to the Interim RPPR. The Interim RPPR (IRPPR) will be used when you are submitting a Competing Renewal application (Type 2). Since the Type 2 application is a competing application, there is no guarantee it will be awarded. And whether it is or isn’t awarded, can create some confusion about what to do for a final report.
So here is the scenario of how the Interim RPPR will be used. If you opt NOT to apply for Competing Renewal, complete the Final RPPR as you normally would within 120 days of the project end date. If you are going to complete a Competing Renewal application (or have already submitted such an application), you will submit an Interim RPPR. This must be submitted within 120 days of the project end date.
If you are awarded the renewal, the Interim RPPR will be treated as your annual RPPR and no other progress reporting will be needed for that segment of the study. If the application is NOT awarded, then the Interim RPPR will be accepted as the Final RPPR.
Like the mystery of how the tribbles were able to consume an entire silo of quadrotriticale (notice it ends in “cale?” Probably just as tasty as kale!), there is still one mystery here as well. Who can initiate and submit these various types of RPPRs? First, annual RPPRs have not changed at all. The Progress Report delegation permits any user with the ASST role the ability to complete progress report information for a specified PI, but they cannot route or submit the report. The Submit delegation permits a specified Principal Investigator (PI) the ability to submit to agency the progress report, listing them as the Signing Official (SO) for that submission.
Second, Interim and Final RPPR work in the same manner as the old Final Progress Report (FPR). With the FPR, both SOs and PIs could initiate and/or submit to agency. They could also route the report back and forth for review and edits. All of this done without the need to delegate any authority. Interim RPPR and Final RPPR work in the same manner. No delegations needed for the initiation and submission of either version of these RPPRs.
Now because the format between the annual RPPR, the Interim RPPR and the Final RPPR are so similar, and permissions and delegations have not changed, this transition will be no tribble at all! (Come on! You knew I was goin’ there!)
No NCE? No Problem!
 You may remember, or if you’re old like me, you might not, an article I wrote back in June of 2015 (really? Was it that long ago??) on expanded authority and No Cost Extensions (“Please Sir, I want some more…”). It basically outlined the process of requesting a No Cost Extension (NCE) when the Extension link becomes available, and what happens if you extend the project before that link is available. Well coming soon for scenarios when the link is not available, Signing Officials will be able to request an NCE electronically through eRA Commons via Prior Approval. Here is how it will work. You will be able to go to the Prior Approval tab in eRA Commons, and from the Initiate a Prior Approval Request box, select No Cost Extension from the drop down and click Go.
You may remember, or if you’re old like me, you might not, an article I wrote back in June of 2015 (really? Was it that long ago??) on expanded authority and No Cost Extensions (“Please Sir, I want some more…”). It basically outlined the process of requesting a No Cost Extension (NCE) when the Extension link becomes available, and what happens if you extend the project before that link is available. Well coming soon for scenarios when the link is not available, Signing Officials will be able to request an NCE electronically through eRA Commons via Prior Approval. Here is how it will work. You will be able to go to the Prior Approval tab in eRA Commons, and from the Initiate a Prior Approval Request box, select No Cost Extension from the drop down and click Go.eRA Commons will find and display a list of eligible grants. Find and select the desired grant and click the Initiate No Cost Extension button. Complete the request form, supplying the requested information.
When is a grant eligible for a NCE through Prior Approval?
- When you have already used a NCE under expanded authority and you are within 90 days of the project end date.
- When you are not under expanded authority and you are within 90 days of the project end date.
- When the project end date has expired and has not been closed or has not entered unilateral closeout, whichever comes first.
When is a grant NOT eligible for a NCE through Prior Approval?
- When you have never requested a NCE under expanded authority and you are within 90 days of the project end date. In this case, the NCE will be processed normally through the Extension link in Status.
- When the grant is closed.
- When the grant is a fellowship grant.
What information will you need to provide?- The NCE request form consists of 4 sections:
- Request Detail – Here you will be asked such things as the number of months you wish to extend the project end date; the amount of unobligated money still available, etc.
- Three PDF upload fields: Progress Report, Budget Document, Justification Document
The exact details of what will be needed in these uploaded files will be outlined by the awarding IC.
The goals here are to standardize the process; provide tracking and accountability for requests; and leverage existing data by working through eRA Commons. But don’t go away! Because “I’ll be back” with even more cool news in the very next article!Prior Approval Changes How PIs Change
 See! I’m back. And I’m happy! And that is because along with the new NCE option in Prior Approval, we are also introducing the ability to request a Change of PD/PI or adding/deleting multiple PIs through Prior Approval. As sometimes happens, institutions need to replace a previously approved Program Director or Principal Investigator (PD/PI) with an individual that is approved by the awarding Institute or Center (IC). This process, starting in late February can be initiated and managed through eRA Commons. Only a Signing Official (SO) can initiate the request. Principal Investigators cannot see Change of PD/PI Requests. The SO logs into eRA Commons; goes to the Prior Approval tab; from the Initiate a Prior Approval Request box; selects Change of PD/PI Request from the drop down; and then clicks Go.
See! I’m back. And I’m happy! And that is because along with the new NCE option in Prior Approval, we are also introducing the ability to request a Change of PD/PI or adding/deleting multiple PIs through Prior Approval. As sometimes happens, institutions need to replace a previously approved Program Director or Principal Investigator (PD/PI) with an individual that is approved by the awarding Institute or Center (IC). This process, starting in late February can be initiated and managed through eRA Commons. Only a Signing Official (SO) can initiate the request. Principal Investigators cannot see Change of PD/PI Requests. The SO logs into eRA Commons; goes to the Prior Approval tab; from the Initiate a Prior Approval Request box; selects Change of PD/PI Request from the drop down; and then clicks Go.The system will present a list of eligible grants. The following conditions must be met for a grant to be eligible for a Change of PD/PI Request:
- The grant has a grant year awarded.
- The grant family is not past the Project Period End Date.
- The grant is not a Fellowship or Career.
- The grant is from an IC/Agency that supports Change of PD/PI using the Prior Approval module.
When a grant is selected from the list, an Initiate Change of PD/PI button is made available. Clicking the button opens the Prior Approval Request Change of PD/PI form. The details for the request require some basic information:
- Who is being replaced, removed/added to the grant?
- What will their level of effort be?
- What is the effective start date for the new PD/PI?
Additionally, some files will be uploaded to be attached to the request.
- Biosketch
- Other Support
- Justification Document
Of course, as with other prior approval requests, working with the Grants Management Specialist of the awarding Institute or Center will be important to know what information will be required for the request.Once the request is submitted, the system creates a PDF of all the submitted information and sends a notification to the SO, the GMS, and Program Officer so they can review the request.Pretty awesome. So hasta le vista, and I’ll be back soon with some more wonderful updates.
Jan 24, 2017
eRA Update: eRA Commons Status Information Issues Resolved
The issues preventing users from accessing some information on the detailed Status Information screen in eRA Commons have been resolved. All data is now being displayed correctly.
(Original message sent Monday, January 23, 2017 at 3:34 p.m. ET)
Jan 23, 2017
eRA Alert: eRA Commons Status Information Issues
Users are experiencing issues accessing some information on the detailed Status Information screen in eRA Commons.
- Study Section Roster —The link to the roster (in the Review section), is resulting in an error for some users.
- Cover Letter — If the application included a cover letter, the cover letter is inaccessible (in the Other Relevant Documents section).
- Scores — Application review scores cannot be accessed by Principal Investigators (PIs).
We are working to actively resolve these issues and will provide more information when it is available. We are sorry for any inconvenience this may cause.
Jan 19, 2017
eRA Update: Termination Notices Available in xTrain
The technical issue with Termination Notices in xTrain has been resolved. Users can once again access and work on Termination Notices.
Original message sent Thursday, January 19, 2017 at 3:07 p.m. ET.
eRA Alert: Termination Notices Temporarily Inaccessible in xTrain
A technical issue with Termination Notices in xTrain is temporarily preventing users from accessing and working on Termination Notices. We are working to correct the issue and should have it addressed shortly. We will update you when more information is available.
We are sorry for any inconvenience.
Jan 17, 2017
eRA Enhancements: eRA Commons Upcoming Release and Scheduled Downtime for Wednesday, January 18
Scheduled Downtime for the January Release:
eRA Commons and ASSIST will be unavailable beginning at 9 p.m. ET Wednesday, January 18 and will return to service by 7 a.m. ET Thursday, January 19, 2017. When the system is available again, you will see new features and functionality have been added to the system.
-
- z edit or add information, the system locks that form so others cannot simultaneously edit the same form.
- ASSIST displays a message indicating the form you are trying to access is locked by another user and now includes the name of the user who holds the lock.
Highlights of eRA Commons January Software Release
- Final RPPR Section D.1 Added
- As part of the transition to the use of the Final Research Performance Progress Report (FRPPR), Section D.1 of Participants is now included.
- Section D.1 asks “What individuals have worked on the project?” This information will be the list of people who have worked on the project since the previous progress report. It does not include all the individuals during the lifetime of the award.
- As part of the transition to the use of the Final Research Performance Progress Report (FRPPR), Section D.1 of Participants is now included.
- New Design of the Status Information Screen for PIs and SOs
The Status Information screen will have a new look and feel. The screen is an important source of information for Principal Investigators (PIs) and Signing Officials (SOs) for such things as scores, summary statements, NIH contacts, reference letter status, etc.
- The new design is similar to other updated screens, such as the Personal Profile and the Institution Profile
- Categories of information are organized into collapsible/expandable sections
- Expand All (default view) and Collapse All buttons show and hide the information on the screen
- New Text Filter
- A new field will allow you to enter text and display the results on those sections with matches
- Matching text will be highlighted, allowing you to quickly locate the information
- Important information is displayed along the left side:
- Warnings
- Contact information
- Scientific Review Officer (SRO)
- Grants Management Specialist (GMS)
- Program Official (PO)
- Latest Updates
- eRA Service Desk contact information
- New Print Capabilities
- New formatting capabilities allow you to print all the information with the click of a single button
- Organized by category
- Includes warnings
- Includes NIH contacts
NOTE: The ability to submit help tickets online will not be available during the downtime listed above.
-
Jan 06, 2017
eRA Information: Ext-UAT and Commons Demo Environment Will be Unavailable Tuesday, January 10, 2017
The External User Acceptance Test (Ext-UAT) and the Commons Demo environment is scheduled to be offline Tuesday, January 10, 2017 from 10 a.m. to 6 p.m. ET in preparation for the winter system-wide software release. As part of our standard practice before each quarterly software release, we update the Ext-UAT and Commons Demo environments with the changes that will take place in production.
These changes will be implemented in production starting on Wednesday, January 18, 9 p.m. ET and ending no later than 7 a.m. ET on Thursday, January 19.
NOTE: Please be reminded this release has been moved up a day earlier than scheduled. The date was moved to ensure functionality will be available for users on Thursday, the day before Inauguration Day.
We are sorry for any inconvenience this may cause. Please note that this process will not affect the production environment.
Jan 03, 2017
Final RPPR
Dec 20, 2016
eRA Reminder: Use of Final RPPR Starts Jan. 1, 2017; Related Changes to IMS
This is a reminder that the Final Research Performance Progress Report is replacing the Final Progress Report (FPR) in eRA Commons effective Jan. 1, 2017. For more details on Final RPPR, please see the November 23rd Notification.
NOTE: For small businesses, the new Final RPPR (or F-RPPR) will be in effect at least 2 months later, due to the unique final reporting requirements that they face under the SBIR/STTR policy directive.
In addition, related changes have been made to the Inclusion Management System (IMS).
What This Means for Inclusion Data Reports in IMS
The ‘last budget period’ Inclusion Data Records (IDRs) will be replaced with ‘final’ IDRs. If IDRs already exist, final IDRs will now be automatically created by the system for the last support year of each competitive segment.
Users will be able to edit existing final IDRs. If additional IDRs need to be created, users can create them using the existing method.
Once the grant is closed, this ability to create or edit IDRs will no longer be available. The Manage Inclusion Data Records (IDRs) screen in IMS will display a ‘Grant Closed’ message. At that point, users will be able to only view final IDRs.
Accessing IDRs for the Final RPPR is the same process as for annual RPPRs. When the Final RPPR is initiated, users will go to Section G.4.b and click on the Inclusion link. This action takes you to the Inclusion Manage Inclusion Data Records (IDRs) screen.
For details, including screenshots, please see the latest updates in the IMS Online Help which will be posted at the end of December.
More Information
See Guide Notice NOT-OD-17-022. You can also visit the NIH RPPR web page, and check out November’s eRA Items of Interest article Please Call it “Final RPPR”.
Dec 08, 2016
eRA Update: Issue with RPPRs Has Been Fixed
The issue affecting Research Performance Progress Reports (RPPRs) in eRA Commons that prevented users from checking for errors and submitting completed RPPRs to the agency has been fixed.
Original message sent Thursday, December 08, 2016 at 3:22 p.m. ET.
eRA Alert: Temporary Issue with RPPRs
An issue has been identified that is affecting Research Performance Progress Reports (RPPRs) in eRA Commons. Users cannot currently check for errors on an RPPR prior to submission, nor can users submit a completed RPPR to the agency. We have identified the cause of the issue and plan to fix this today.
We are sorry for any inconvenience.
Dec 06, 2016
eRA Information: Quarterly Release Date for Commons, ASSIST and IAR Moved to Jan. 18, 2017
eRA’s winter release will be moved to Wednesday, January 18, 2017, a day earlier than scheduled.
The date was moved to ensure functionality will be available for users on Thursday, the day before Inauguration Day.
During this system-wide release, eRA Commons, ASSIST, IAR and related services are scheduled to be unavailable starting at 9 p.m. on Wednesday, January 18 and ending no later than 7 a.m. on Thursday, January 19.
NOTE: You can review all the release and maintenance dates by checking eRA’s Deployment and Maintenance Calendar, which is updated on a regular basis. You can add this calendar to your own calendar by clicking on the icon on the calendar page.
eRA Enhancements: New Features for xTRACT Coming December 8, 2016
In a software release scheduled for Thursday, December 8, 2016, several new features will be added to xTRACT. xTRACT is the Extramural Trainee Reporting And Career Tracking system and is accessed via eRA Commons. It allows applicants, grantees and assistants to create research training tables for progress reports and institutional training grant applications.
Features
Ability to copy data from a recently prepared RPPR RTD into a Renewal RTD
When working with a Renewal Research Training Dataset (RTD), xTRACT users will now be able to copy Participating Trainee and Program Statistics data from a recently prepared RPPR RTD for that same training grant. If an RPPR RTD for the grant’s final non-competing period has already been started, then the data will be copied from that. Otherwise, if an RPPR RTD for the grant’s previous non-competing period is available, the data will be copied from that RTD instead.
The option to copy data will be offered when the Renewal RTD is first initiated. Users will also have the option to copy each type of data (Participating Trainee or Program Statistics) when editing the RTD, whenever visiting each of the corresponding data entry sections.
Ability to copy data from a recently prepared Renewal or RPPR RTD into an RPPR RTD
When working with an RPPR RTD, xTRACT users will now be able to copy Participating Trainee and Program Statistics data from a recently prepared RTD for that same training grant. A copy can be performed from the previous year’s RPPR RTD for that grant. Also, when preparing the RPPR RTD for the final non-competing year, there will be an additional option to copy from the next Renewal RTD for that grant (provided the Renewal RTD has been started).
The option to copy data from a recent RTD will be offered when the RPPR RTD is first initiated. Users will also have the option to copy each type of data (Participating Trainee or Program Statistics) when editing the RTD, whenever visiting each of the corresponding data entry sections.
Improved Performance of Preview/Finalize RTD
The amount of time required to preview or finalize an RTD has been improved. When choosing either one of these options, results should appear more quickly than before.
Fixes
Adjustment to the auto-population of Faculty Research Support, from NIH and Other Agency Sources
Faculty Research Support from NIH and Other Agency grants are automatically populated in xTRACT, based on award data that we have on hand (and cannot be edited). However, there have been some occasions when an NIH/Other Agency grant has not been listed – even though it seems like it should have appeared. Often when this occurred, it was because of a short delay in funding the most recent noncompeting year for the research grant in question, making it appear that the grant was not active at the time that the RTD was being prepared. An adjustment has been made to account for this sort of timing, so that any such awards are correctly included in the listing of NIH/Other Agency Research Support for that faculty member.
Applicants and Entrants Section
Some users had noted cases where multiple occurrences of the same “Prior Institution” would appear on rare occasions, after entering these in the Applicants and Entrants section. This problem has been resolved.
Following the release, please look for details and screenshots in the Online Help for xTRACT (and accessible through the question marks on xTRACT screens).
Nov 29, 2016
eRA Final Reminder: Update Your Browsers by Nov. 30 to Continue Using eRA Modules
This is a final reminder that on Wednesday, November 30, 2016, eRA is updating access to all its publicly-accessible websites to use ‘https only’ secure connections per federal mandate (OMB M-15-13, dated June 8, 2015). Please update your browsers to continue using eRA Commons, ASSIST, IAR and iEdison. NOTE: This update will not impact System-to-System connections or security certificates.
Original message below sent Wednesday, October 26, 2016 at 8:26 a.m. ET.
Nov 23, 2016
eRA Information: Final RPPR To Be Used Effective Jan. 1, 2017
The Final Research Performance Progress Report (F-RPPR) will replace the Final Progress Report (FPR) for grants closeout, effective January 1, 2017. The F-RPPR will be available for use in eRA Commons on January 1, 2017.
NOTE: For small businesses, the new F-RPPR will be in effect at least 2 months later, due to the unique final reporting requirements that they face under the SBIR/STTR policy directive.
What This Means for You
If you have a final progress report due, and you wish to use the old FPR format of an uploaded document, you must submit the FPR before January 1, 2017. NIH will no longer accept any of the old format FPRs on or after January 1, 2017.
The Format
The format of the Final RPPR is very similar to that of the annual RPPR. The notable differences being the F-RPPR only uses section D.1 for Participants, and does not use sections F (Changes), and H (Budget). The F-RPPR does have a new section: Section I (Outcomes).
Project Outcomes (Section I) will be made publicly available, allowing recipients the opportunity to provide the general public with a concise summary of the public significance of the research.
Deadline Remains Unchanged
The deadlines for submitting a Final RPPR remain the same – no later than 120 days from the project end date.
The Effect on Delegation to Submit RPPR
NIH will maintain the business rule that allows the Signing Official (SO) to delegate the submission of the Final RPPR or Interim-RPPR to a Program Director/Principal Investigator (PD/PI).
More Information
See Guide Notice NOT-OD-17-022. Visit the NIH RPPR web page, and check out November’s eRA Items of Interest article Please Call it “Final RPPR”.
Nov 22, 2016
eRA Update: eRA Commons and ASSIST Connection Issue Has Been Resolved
The issue affecting user connections to eRA Commons, ASSIST, iEdison and related services has been resolved. Original message sent Tuesday, November 22, 2016 at 10:51 a.m. ET.
eRA Alert: eRA Commons and ASSIST Users Experiencing Connection Issues
The eRA Service Desk has received reports that users of eRA Commons, ASSIST, iEdison and other related services are inadvertently being logged off or unable to log in. The problem has been identified as an NIH wide issue. We will provide more information when it is available. We are sorry for any inconvenience it may cause.
Nov 21, 2016
eRA Update: Issue with Entering Publications for Trainees in xTRACT Has Been Resolved
The issue keeping users from adding publications for Trainees in xTRACT has been resolved. Original message sent Thursday, November 17, 2016 at 4:22 p.m. ET
Nov 17, 2016
eRA Alert: Temporary Issue with Entering Publications for Trainees in xTRACT
Users are unable to add publications for Trainees in xTRACT currently, because of an issue with PubMed searches. Most users add their citations by searching for them in PubMed first. The issue is being addressed and should be resolved shortly. We will update you as soon as we are informed it has been fixed. We are sorry for any inconvenience.
Nov 15, 2016
eRA Reminder: Please Update Your Browsers by Nov.30 to Continue Using eRA Modules
Just a reminder that on Wednesday, November 30, 2016, eRA is updating access to all its publicly-accessible websites to use ‘https only’ secure connections per federal mandate (OMB M-15-13, dated June 8, 2015). Please update your browsers to continue using eRA Commons, ASSIST, IAR and iEdison. NOTE: This update will not impact System-to-System connections or security certificates.
Original message below sent Wednesday, October 26, 2016 at 8:26 a.m. ET.
Nov 09, 2016
NIH eRA Items of Interest — November 2016
 And not “FRIPPER!” It just starts the earworm of the theme song from ‘Flipper’ burrowing into my mind...
And not “FRIPPER!” It just starts the earworm of the theme song from ‘Flipper’ burrowing into my mind...If you were lucky enough to attend the NIH Regional Seminar conference in Chicago last month, you may have heard Scarlett Gibb, Customer Relationship Manager for eRA Commons, discussing the new Final RPPR.
Tentatively scheduled for required use as of January 1, 2017, the Final RPPR report will replace the current Final Progress Report for Closeout. As you know, the Final Progress Report is not strictly formatted, and basically has some half a dozen topics that need to be addressed, including a statement of progress; list of significant results, inclusion report, if applicable; list of publications; as well as any award specific instructions. The report is then uploaded as a PDF through eRA Commons and submitted to the agency by the signing official.
As part of Uniform Guidance (UG), we transitioned to the Research Performance Progress Report (RPPR) back in October of 2014.
So, come 2017, the RPPR format will be extended to the Final Progress Report. One of the differences between RPPR and the Final RPPR is that not all sections will be part of the final report. For example, Section D – Participants; Section F – Changes; and Section H – Budget will not be part of the Final RPPR. Plus, instead of a PDF upload, the information will be entered into RPPR-like screens. The new screens will include a new Section I – Outcomes.
The transition date from the current Final Progress Report Process to the Final RPPR will be a strict one. The anticipated plan specifies that if you have a progress report due, and you want to use the old format, it must be submitted prior to January 1, 2017. Any final progress report submitted after January 1, 2017 will need to be submitted as a Final RPPR. Any other submission format will be rejected and you will need to resubmit in the Final RPPR format.
This is just an introduction to what is coming so you can be ready. And now a brief musical interlude… “They call him Flipper, Flipper, faster than lightning, No-one you see, is smarter than he, and we know Flipper…”
The World is Not Flat, But Status Is (or Can Be)
 How many of you remember this little guy from your high school biology class? Oh, wait, look whom I’m asking! But flatworms aren’t alone in being flat. Now your Status Results screen can be flat too!
How many of you remember this little guy from your high school biology class? Oh, wait, look whom I’m asking! But flatworms aren’t alone in being flat. Now your Status Results screen can be flat too!You may remember that early this year, January 22, to be exact, we introduced a new view of the Status Results screen for Principal Investigators (PIs). This new design grouped applications and awards into families. These groups can be expanded with a click, or left collapsed, giving you a more concise view of your data. The design is responsive, adjusting to various window sizes, and recently color coded to provide the most recent status of an application.
And now a new feature has been added. A set of buttons in the upper right corner lets you toggle between the newer look (Grouped View) and a more traditional look (Flat View). In the Flat View you will see all of your applications and awards listed, organized in the traditional manner of columns: Application ID, Grants.gov Tracking #, Proposal Title, etc.
So not only is the world round, but now you get the best of both of them…Well, in Status Results anyway!My work is not yet done!They say I must upgrade as fast as I can, otherwise Commons may not run.The time is near, warnings I hear, November 30th fast approaching,HTTPS to be secure, my browser I must be preparing;But O Chrome! IE! Firefox!
Supported you are by this Fed Versions I must check else my browser be dead.Just a little nod to Walt Whitman as a reminder to check that you are using a compatible browser as eRA transitions to meet federal security guidelines. For more information, see the communication sent on October 26, 2016. A recent addition to eRA Commons has been the Prior Approval feature. Currently found on the main navigation bar, Prior Approval supports two processes that have gone electronic: The request for withdrawal of an application (see Guide Notice NOT-OD-16-143); and the request to a granting agency to submit an application with direct costs of $500K or more per year (see Guide Notice NOT-OD-17-005). This latter process is sometimes referred to as a $500K request. These electronic processes through Commons are currently optional and not yet required.To help you understand how each of these work, we have released two new tutorial videos on our eRA Videos page. On the page, look for the eRA Commons: Prior Approval link. This will take you to the section of the page for the two videos.
A recent addition to eRA Commons has been the Prior Approval feature. Currently found on the main navigation bar, Prior Approval supports two processes that have gone electronic: The request for withdrawal of an application (see Guide Notice NOT-OD-16-143); and the request to a granting agency to submit an application with direct costs of $500K or more per year (see Guide Notice NOT-OD-17-005). This latter process is sometimes referred to as a $500K request. These electronic processes through Commons are currently optional and not yet required.To help you understand how each of these work, we have released two new tutorial videos on our eRA Videos page. On the page, look for the eRA Commons: Prior Approval link. This will take you to the section of the page for the two videos.The videos themselves are shared from YouTube. But please do not bookmark the YouTube links. Since these videos will eventually be updated, the YouTube URLs will become invalid. To make sure you always have access to the latest tutorials, you can bookmark the eRA Videos page for all our videos, or the specific section (in this case, the eRA Commons: Prior Approval link), or you can even link to the specific part of the page for each video:
So call the gang together, pop some popcorn, and enjoy these latest releases!
Oct 26, 2016
eRA Enhancement: Redesigned Section in RPPR to Help Grantees Categorize Products Developed Under a Grant
Grantees filling out a Research Performance Progress Report in eRA Commons are expected to list significant products developed with their grant in Section C of the RPPR, namely websites, technology and other products.The product sections of the RPPR have been redesigned recently to help grantees better categorize the products arising from their grant. A new scrollable menu lists 14 product categories under which to list the product(s): Audio or video; data or databases; research material; educational aids or curricula; evaluation instruments; instruments or equipment; models; physical collections; protocols; software; survey instruments; interventions (e.g. clinical or educational); new business creation and other.After entering a description for one or more reportable products in a text box, the grantee can then select appropriate categories for the product using a scrollable menu. Grantees are not limited to a single category per product; please select as many categories as appropriate for the product being reported.In addition, grantees can turn to a new resource ‘Guide to Categorizing Products in RPPR’s Section C’ to find definitions, examples and distinctions to help them decide which categories are appropriate for their product(s). For instance, videos developed to elicit behavioral change, such as counseling or motivational videos, or to instruct patients, may also meet the definition of clinical intervention. Figure 1: Screenshot of a subsection of RPPR Section C, displaying the scrollable menu on the left.This menu can be found in all three subsections of Section C: websites (C.2), technology (C.3) and other products (C.5). Grantees who do not have a product to report can click on the ‘Nothing to Report’ checkbox.
Figure 1: Screenshot of a subsection of RPPR Section C, displaying the scrollable menu on the left.This menu can be found in all three subsections of Section C: websites (C.2), technology (C.3) and other products (C.5). Grantees who do not have a product to report can click on the ‘Nothing to Report’ checkbox.eRA Information: Please Update Your Browsers by Nov. 30 to Use eRA Modules; New Security Mandated For Websites
eRA has been working on strengthening the security of its modules, web services and websites to the federally mandated ‘https only’ secure connection required for all federal agencies.
The ‘https’ connection offers “the strongest privacy and integrity protection currently available for public web connections,” the Office of Management and Budget wrote in mandating federal agencies adopt this standard by the end of 2016.
Effective November 30, 2016, all eRA modules (eRA Commons, ASSIST, IAR and iEdison), web services and websites will use ‘https only’ secure connections.
What This Means for You
You may need to update your web browser to continue using eRA modules:
- With this policy, some browser versions will no longer work.
- Here is a list of browsers and versions that will still work properly following the update:
These three are also the browsers eRA uses to develop and test its modules for browser compatibility, as listed in the eRA Browser Compatibility statement. While lower versions are listed below, we encourage you to upgrade to the versions listed in our browser compatibility statement above to ensure you have an optimal experience when using eRA modules.
- Google Chrome® version 4.0.211.0 and higher
- Firefox® version 4 and higher; with Firefox 17, Mozilla® integrates a list of websites supporting the new protocol
- Internet Explorer® 11 on Windows® 8.1 and Windows® 7 when KB 3058515 or higher is installed (Released on Windows Update in June 2015)
These four are the other browser versions that will work with the security upgrade, but are not included in the in the eRA Browser Compatibility statement:
- Chromium®
- Opera® version 12 and later
- Safari® as of OS X Mavericks
- Microsoft® Edge™ and Internet Explorer® 11 on Windows® 10
More information is available at https://https.cio.gov/ and OMB M-15-13.
Oct 18, 2016
eRA Enhancements: eRA Commons’ Upcoming Release and Scheduled Downtime for Thursday, October 20, 2016
Scheduled Downtime for the October Release:
eRA Commons and ASSIST will be unavailable beginning at 9 p.m. ET Thursday, October 20, and will return to service by 7 a.m. ET Friday, October 21, 2016. When the system is available again, you will see new features and functionality have been added to the system.
Highlights of eRA Commons’ October Software Release
- New Non-Research Tab Has Been Added to the Top Navigation
- As part of the expansion of eRA services to other federal agencies, the Non-Research tab has been added for recipients of SAMHSA (Substance Abuse and Mental Health Services Administration) non-research grants.
- The new tab is located after eRA Partners.
- Only those of you who also receive SAMHSA non-research grants will need to access this tab to manage post award amendments.
- SAM Registration Expiration Data Displayed
- The expiration date for institutions’ System for Award Management (SAM) registration will now be displayed on the Institutional Profile,
- The SAM registration needs to be renewed yearly and is required to successfully submit a federal grant application to Grants.gov.
NOTE: The ability to submit help tickets online will not be available during the downtime listed above.
- New Non-Research Tab Has Been Added to the Top Navigation
Oct 14, 2016
NIH eRA Items of Interest - October 2016
Prior Approval – $500K
A principal investigator (PI) needs to seek prior approval from the NIH before submitting a grant application with direct costs of $500K or more for a single budget year.
NIH has been developing a way to provide you with an option to electronically submit these prior approval requests through eRA Commons. And effective Sept. 15, this option is a reality.
Here is how it works:
The Invite to Initiate a $500K Request
The PI will reach out via email or phone to the Program Official at the Institute/Center (IC) with whom they have been working concerning the $500K request, per current practice. The PO can then choose to invite the PI to initiate the prior approval request through eRA Commons. The initiation of the request will trigger an email notification to the PI and to the email address listed for receiving the Notice of Award (NoA) on the Institutional Profile screen.PI Action
Upon being notified, the PI will go into eRA Commons and go to the Prior Approval tab along the top navigation menu. The PI will find two options and should click List my Requests. The PI will find the $500K Request under the column Request Type, with a status of “In Progress PI,” and should click the “Modify” link.The Prior Approval Request $500K screen will open. The screen is pretty straightforward with a few required fields, such as Project Title, FOA number, and Anticipated Submission Date. The PI will need to provide a short justification (just 500 characters) for the request, with up to 10 supporting documents allowed. Depending on the business processes of the institution, the PI can route the request to the SO for review, or submit directly to NIH.
SO Action
Since the notice to submit the prior approval request is sent to the NoA address as well, the SO should login to eRA Commons and go to the Prior Approval tab. SOs should use the Search for Requests button and select the $500K Request under the Request Type drop down. The SO has the ability to view the request, or if they choose, recall it, thus giving them the ability to modify it and submit it.Next Steps
If the request is approved by the Program Official at the IC, the PI will receive an email from the Program Official. When the error free application is received by NIH, this application will be matched with the $500k approval from the IC and the application will move through the normal process.For the moment, this is an option for the submission of $500K requests. However, as we continue to move from all paper processes to a formal electronic environment, this option may become a requirement as we seek solutions that provide accountability, transparency and improved reporting capabilities.
There are other features (status and history), which you can read about in the Prior Approval section of the eRA Commons Online Help.
For more information, please see Guide Notice NOT-OD-17-005.
Also, coming soon will be two new video tutorials on Prior Approval. One focuses on the request to withdraw an application, and the second demonstrates the process of $500K requests as described above.Password and Login Issues
True story: I woke up the other morning and received an alert that one of my bank accounts had dipped below the minimum threshold. That’s not supposed to happen. I panicked.
I went to log in to see what was happening. With hands shaking, blood pressure rising, fear running down my spine, I was greeted with “Invalid password. ACCESS DENIED!” Then I freaked! Then my dog freaked. And my wife? She just walked away (such a smart woman). Then I remembered that I had recently changed that password. Whew!
Oh, passwords… such a necessary scourge. You need a certain number of characters; special characters; numbers; capital and lower case letters; they expire every 32 seconds; you can’t use the last 311 passwords; etc. But where would we be without them?
And of course for eRA Commons, we have similar requirements for passwords. So is it any wonder the most frequent calls to the eRA Service Desk are about passwords and trouble logging in? Not really.
So some colleagues and I got together and developed a kind of troubleshooting support document for login issues, cleverly titled, ‘Having Trouble Logging in to eRA Commons?’ This walks you through the four most common issues related to logging into eRA Commons. And then on the back (also cleverly titled, ‘Additional Information and Resources Concerning Login Issues’) is a list of other errors, Password Guidelines, and how to contact the eRA Service Desk.
For example, did you know your Commons password has to have a minimum of 8 characters, BUT it cannot be more than 16 characters? Or that you cannot use any of your previous 24 passwords? See, helpful information.
Take a look, download it, and share it with friends and colleagues. Hopefully it can help you avoid a moment of panic! And as it turned out, my panic was only kind of justified. My A/C company errantly charged me twice for my service contract. The issue was resolved by 9AM. And my wife came back.
Oct 13, 2016
eRA Information: Ext-UAT and Commons Demo Will be Unavailable Friday, October, 14 2016
The External User Acceptance Test (Ext-UAT) and the Commons Demo environments are scheduled to be offline Friday, October 14, 2016 from 10 a.m. to 6 p.m. ET in preparation for the fall system-wide software release. As part of our standard practice before each quarterly software release, we update the Ext-UAT and Commons Demo environments with the changes that will take place in production.
These changes will be implemented to production starting on Thursday, October 20, 2016 at 9 p.m. ET.
We are sorry for any inconvenience this may cause.
Please note that this process will not affect the production environment.
Sep 09, 2016
eRA Enhancements: Electronic Requests for Withdrawal of Applications Now Available Through eRA Commons
A new feature was added to eRA Commons during a software release yesterday, Thursday, September 8, 2016. This feature provides Signing Officials the capability to electronically submit a request to withdraw an application.
After the successful submission of an error free application, the Signing Official (SO) has two business days to reject an application. After those two days, the SO must contact the Division of Receipt and Referral (DRR) to request to withdraw the application. This latter process can now be accomplished electronically through eRA Commons.
The submission of an electronic request for withdrawal is available as long as the application has not gone to review and the summary statement posted. At that point the application cannot be withdrawn.
Features
- The Initiation of a Withdrawal Request Can Be Done by the SO or PI
- The SO is the only person who can request that NIH withdraw an application.
- The PI can request that the SO do so by initiating the withdrawal request through Commons, providing justification (required) and relevant supporting documentation (a maximum of 10 pdfs of up to 5 MB each can be uploaded).
- The PI must then route the request to an SO at their institution for review and submission to NIH.
- The SO may make the withdrawal request even if the PI has not initiated the request.
- The SO may check the status of the withdrawal request by contacting DRR.
- A New Navigation Tab, Prior Approval, Added to Commons
- The Prior Approval tab opens a new screen that allows both PIs and SOs to initiate requests or review for existing requests.
- To initiate a Withdrawal Request, from the Initiate a Prior Approval Request drop down, select Withdrawal. The system will display grants eligible to be withdrawn.
- The List my Requests screen will display:
- The request ID (system generated ID number)
- Request Type
- Prior Approval Status (In Progress PI, In Progress SO, Submitted to Agency)
- Application ID
- Project Title
- Action (Modify or View depending on status)
- SOs Have the Ability to Search for Requests
- For the SO, the List My Requests option will display any requests that are pending their approval.
- SO can also use the Search for Requests button to find those withdrawal requests that have been submitted or have a status of “In Progress PI,” which means the PI has initiated the withdrawal but may still be working on it, and has not routed it to the SO.
- Automated Notifications
- Upon submission, both the submitting SO and PI will receive notification.
- Upon approval of withdrawal by DRR, the PI will receive a notification. The SO will see the application status has changed to Withdrawn, and will see the withdrawal confirmation in the Correspondence section of the Status Information screen in eRA Commons
NOTE: Applications with a status of “To be Paid” or “Awarded” cannot be withdrawn in this manner.
For more information concerning Prior Approval for Withdrawals, please see Guide Notice NOT-OD-16-143 and the Prior Approval section in the eRA Commons online help.
- The Initiation of a Withdrawal Request Can Be Done by the SO or PI
Aug 16, 2016
eRA Enhancements: Improvements to xTRACT To Be Implemented August 18, 2016
In a software release scheduled for Thursday, August 18, 2016, several new features will be added to xTRACT. xTRACT is the Extramural Trainee Reporting And Career Tracking system and is accessed via eRA Commons. It allows applicants, grantees and assistants to create research training tables for progress reports and institutional training grant applications.
Features
- Publication citations may now be searched and imported from Pubmed.
When entering publications for a participating trainee, xTRACT now provides users with the ability to search Pubmed using either the Pubmed identifier, the author name, or publication title. The ability to enter a full citation manually (instead of searching Pubmed) is still available as well, for any occasion where the user may require it.
- Access for delegated assistants (users with ASST role) is expanded.
Assistants may now perform the following on behalf of their PIs:
- Initiate the Research Training Dataset (RTD) for New Applications
- Finalize an RTD
- Unfinalize an RTD
- Sections labeled “NIH Support” are now labeled “NIH and Other Agency Sources of Support on Record.”
This labelling more accurately reflects xTRACT’s ability to present grants from some other (non-NIH) agencies for selection, when citing support information for faculty and trainees.
- Table 4’s column labeled “Funding Source” now interprets the funding agency.
When previewing and finalizing the PDF of the RTD, any sources selected from the xTRACT database will display the funding source accordingly:
- If the funding is affiliated with the NIH, displays "NIH"
- If the funding is affiliated with the AHRQ, displays "AHRQ"
- If the funding is affiliated with any other agency or HHS Operating Division, displays "Other Fed”
- Computed means across all years are now displayed to one decimal point.
In Table 6B the calculated mean value for Counts were previously rounded to the nearest whole number. These values are now computed to one decimal point precision and rounded to that decimal place.
- Bookmarks now included in PDFs.
When previewing and finalizing the output PDF, bookmarks are now included to provide navigation to the beginning of each training table.
Fixes
- Corrects an earlier defect in Table 8 generation, where the same source of support might occasionally be cited twice for the same training year.
- Improved performance/response time when navigating to the list of Participating Faculty for an RTD.
Following the release, please look for details and screenshots in the Online Help for xTRACT (and accessible through the question marks on xTRACT screens).
Jul 20, 2016
eRA Enhancements: eRA Commons’ Upcoming Release and Scheduled Downtime for Thursday, July 21
Scheduled Downtime for the July Release:
- eRA Commons and ASSIST will be unavailable beginning at 9 p.m. ET Thursday, July 21, and will return to service by 7 a.m. ET Friday, July 22, 2016. When the system is available again, you will see new features and functionality have been added to the system. The following are highlights of eRA Commons’ upcoming July software release.
- Highlights of eRA Commons’ July Software Release
- Updates in eRA Commons
- NIH has added new features to eRA Commons:
- New ASSIST Tab for Signing Officials (SO) and Principal Investigators (PI)
- As part of the top navigation menu, an ASSIST tab will be available to SOs and PIs
- The new tab will be located between the Status tab and the Prior Approval tab
- Selecting the tab will launch ASSIST in a new window and automatically authenticate the user, providing immediate access to ASSIST
- New RPPR Feature for Publications
- When selecting/deselecting publications for RPPR submissions, users will see a gold lock icon next to those publications which cannot be removed from the RPPR
- To remove the grant affiliation from the publication with a gold lock, users will need to contact the NIHMS help desk through their web form, which is accessible at http://www.nihms.nih.gov/.
NOTE: The ability to submit help tickets online will not be available during the downtime listed above.
Jul 18, 2016
eRA Information: Ext-UAT and Commons Demo Will be Unavailable Monday
The External User Acceptance Test (Ext-UAT) and the Commons Demo environments are scheduled to be offline Monday, July 18, 2016 from 9 a.m. to 6 p.m. in preparation for the summer system-wide software release. As part of our standard practice before each quarterly software release, we update the Ext-UAT and Commons Demo environments with the changes that will take place in production. These changes will be implemented to production starting on Thursday, July 21, 2016 at 9 p.m. ET. We are sorry for any inconvenience this may cause. Please note that this process will not affect the production environment.
Jul 01, 2016
NIH eRA Items of Interest — July 2016
Who’s Who? AMS Answers That Question

So here is a challenge for research administrators… How do you get a list of all the people affiliated with your institution that:
Have an eRA Commons account
Have a role of PI, and/or
Have a role of IAR?
It’s enough to make your eye twitch (see pic) and your brain hurt. Or at least it was.Now as part of eRA Commons, on the Account Management tab you can search for just this kind of information. But wait, there’s more!
Not only can you search for this information, you can export it as well! When you go to the Admin tab>Accounts>Account Management, eRA Commons will open a new window. Here, under AMS (Account Management System), you will see two options: Manage Accounts and AMS User Reports. It is the AMS User Reports that will make your life infinitely easier.
AMS User Reports allows you to search for users affiliated with your institution based on eRA Commons roles. That would be all 19 eRA Commons roles, scientific and administrative. The search is inclusive, meaning that if you search for, say an Account Administrator (AA) and an Assistant (ASST), the results will include users with either one of those roles or both of those roles.
Once the results are returned, the system defaults to showing 10 results per page, but this can be changed to 25, 50 or All. And unlike account searching that is limited to returning a maximum of 500 results, there is no limit to this search. It is already restricted to only search your institution, not all of eRA Commons.
Now how do you get those results in some kind of shareable format? Easy. You will also see two buttons: Export to Excel, and Export to PDF. Awesomely self-explanatory here… clicking either of these buttons will download the results in the format selected. BOOM! Done! (mic drop).FCOI Confusion?

Christmas morning growing up was always a huge event, like for so many kids. In our house, my two older brothers and I would wake up well before dawn and anxiously await for our parents to roll out of bed. Then when we got the OK, we would race down the stairs. Santa always left our presents in individual piles, one for each of us. When I was 5 years old, I remember being so confused because the piles for myself and my oldest brother were small and consisted of socks, underwear, a toothbrush, etc. While Sean’s pile was almost as high as the tree and was made up of an Erector Set, Hot Wheels, GI Joes, etc. and no socks, and no underwear.
And sometimes we do this to you too. We surprise in the most unexpected ways. Which sometimes leaves you confused. Case in point, we recently added a new field to the Institutional Profile form for Financial Conflict of Interest (FCOI) email address. This is found in the Institution Contact Information section. In most institutions, the people responsible for addressing financial conflicts are less directly involved in the grant process and so, over the years we had received numerous requests to have a place to put an email address for those folks. So, we did.
Now it is a required field, and we needed to put something in there for all the preexisting institutions at the time of the update. After some debate and discussion, it was decided that the field would be prefilled with the same email address as the Notice of Award (NoA) email field.
Here comes the surprising and confusing part. Some of you are now asking why your NoA folks are getting FCOI emails. And now you have the answer. The fix however, is easy. Update your Institutional Profile form with the desired email address for Financial Conflict of Interest related communications, and then sigh a deep breath of relief.
Just as I did when my parents, momentarily astounded, said that Santa must have made a mistake as they quickly rearranged the piles for a more equitable distribution of toys. Of course, Santa’s mistake was not realizing my brother Sean would sneak downstairs in the middle of the night and rearrange the piles!Blowing into the Windy City: NIH Regional 2016

What? So I like cartoons (and dinosaurs). Don’t judge me.
NIH is hitting the road again! This time in the Windy City, Chicago, IL , October 26-28, 2016. After a wonderful regional seminar in Baltimore in May, we are ready to do it again. This time I am looking forward to deep dish pizza, maybe a Cubbies game, or seeing The Bean! Registration for Chicago is as easy, so sign up soon! The agenda is full of great learning and sharing opportunities, and, of course I will be there with a bunch of wonderful NIH staff who will also be conducting the ever popular 1:1 sessions. Like the loveable troupe of intelligent dinosaurs that come from the past to educate folks of modern day, we come to help you learn about NIH’s grant processes. And we promise to fill up on our Brain GrainHave a wonder July 4th Holiday!
Jun 23, 2016
eRA Update: Issue with PRAM Functionality Resolved
The issue preventing the successful submission of Progress Report Additional Materials (PRAM) requests has been resolved.
Original message sent Monday, June 20, 2016 at 11:53 A.M.
Jun 22, 2016
eRA Information: NIH Maintenance This Weekend May Affect Connectivity in eRA Modules
NIH will be conducting required maintenance on all of its systems this weekend, Saturday, June 25 and Sunday, June 26 between 10 a.m. and 6 p.m. on both days. As a result of this NIH-wide maintenance, eRA systems may experience intermittent connectivity issues. Users logged in at the time of the maintenance may be affected and will need to log in again.
We are sorry for any inconvenience.
Jun 20, 2016
eRA Alert: Issue with PRAM Functionality
An issue has been found where users are unable to submit a Progress Report Additional Materials (PRAM) request. After clicking submit, the status remains “System is processing your submission” and no indication of a successful submission is displayed. However, the PRAM is saved and will be available for submission once this issue is resolved.
The issue is being addressed and should be resolved shortly. We will provide more information when it is available. We are sorry for any inconvenience it may cause.
May 23, 2016
eRA Enhancement: ASSIST’s New SAM Status Notification Coming May 24, 2016
Application Submission System & Interface for Submission Tracking (ASSIST) will be upgraded on Tuesday, May 24, 2016. As part of the upgrade process, a new feature will inform users of their SAM registration status.
System of Award Management (SAM) is one of the required registrations for applying to and receiving federal grants. However, SAM registrations expire and need to be renewed yearly. As part of ASSIST, a pop-up message window will appear if the registration is going to expire within 14 days or has expired. The pop-up message window will appear when an opportunity is initiated, and when the application status is set to “Ready to Submit.”
In addition to the pop-up message window, a new status button will be added to the initiation page for all new applications. Users will be able to confirm the status of their institution’s SAM registration by clicking this button. Should you have questions concerning the SAM registration, you can get additional information at www.sam.gov. SAM help is provided by the Federal Service Desk at fsd.gov.
NOTE: All eRA systems will remain available during this upgrade process.
Apr 29, 2016
eRA Information: Highlights Coming to eRA Commons’ xTRACT
xTRACT, accessed via eRA Commons, allows applicants, grantees and assistants to create research training tables for progress reports and institutional training grant applications.
Features
The features below were highlighted in the April 11, 2016 communication. For detailed information on these changes, please go to: https://era.nih.gov/news_and_events/news_eRA.cfm#04112016
- Bold Trainee Names: Users will have the ability to “bold” the trainee name within a publication citation, enabling them to comply with the instructions for table 5 (publications). This will be accomplished by using HTML bold tags ( <b>name</b> ) when entering the name of a trainee in the list of authors for a publication.
- Editable selection fields: When adding institution names and departments on post-training positions and for prior institutions for Applicants and Entrants, users will have the ability to type in the name and save the information directly to the field.
- User Interface (UI) improvements: xTRACT will use type-ahead drop-down lists (also referred to as “predictive search”) in certain places. This means as the user begins to type in the field, the system immediately displays matching results, and thus reducing the time it takes to find the desired result.
In addition to the features listed above, the following improvements are scheduled for the Thursday, April 28, 2016 release:
- Simplified Data Entry for Participating Departments and Programs: A single button is now available to add either a Department or a Program. When a Department or Program is added to the RTD, the list results now include a column to indicate specifically whether it is a department or program.
- Revised Left-Navigation Options for Institution Data: The left-navigation menu is now simplified to exclude the “create” options. The ability to create a new program is now provided within “Maintain Programs”. Likewise, the ability to create a new funding source is now provided within “Maintain Funding Sources”.
- Maintain Programs now presents the list of programs without requiring a search: The Maintain Programs screen is now pre-populated. The search dialogue has been removed so whenever a user visits Maintain Programs, the entire list of programs for the organization is shown by default. If desired, the list can be filtered to find specific programs using the “Filter” control at the top of the list.
- Fields support additional digits: Both the Direct Costs on Faculty Non-NIH Research Support, and Months of Research Experience in Applicants and Entrants Section, now support an additional digit. Direct Costs has been expanded from 6 digits to 7, while Months of Research Experience has been expanded from 2 digits to 3.
Fixes
- Same NIH grant support shows multiple times for the same faculty member in Table 4: In some cases the same NIH grant support was showing up multiple times for the same faculty member, when table 4 was rendered in the PDF. This problem has been resolved so that the support only shows once.
- Population of NIH Research Support for faculty should exclude training mechanisms: Per table 4 instructions, xTRACT should be excluding institutional research training grants, institutional career development grants, and research education grants when listing NIH Research Support for faculty. This problem has been resolved so that only research support is shown and the following activity codes are excluded: T15, T32, T34, T35, T90, TL1, TL4, K12, KL2, R25, R90, RL5, RL9, D43, D71.
- In some cases, the awarded trainee positions in the Appointment page on the UI, and in the PDF for table 7 were showing incorrectly: This has been resolved, and the number of awarded positions is now showing correctly in these cases.
- In some cases, a large number of prior institutions in the Applicants and Entrants section was causing the Preview PDF to stall: This issue has been resolved.
For more information about what’s new in xTRACT, check out xTRACT Online Help.
Apr 25, 2016
eRA Update: Issue with PRAM Functionality Resolved
An issue affecting Progress Report Additional Materials (PRAM) links for both Agency PRAM requests and Public Access PRAM requests has been resolved.
Original message sent Friday, April 22, 2016 at 2:56 p.m.
Apr 22, 2016
eRA Alert: Issue with PRAM Functionality
An issue has been found when Principal Investigators (PIs) access Progress Report Additional Materials (PRAM) links for both Agency PRAM requests and Public Access PRAM requests. Clicking the link results in an error message. The issue is being addressed and should be resolved shortly. We will provide more information when it is available.
We are sorry for any inconvenience it may cause.
Apr 18, 2016
eRA Enhancements: eRA Commons’ Upcoming Release Scheduled Downtime for Thursday, April 21
Scheduled Downtime for April Release:
eRA Commons, ASSIST and iEdison will be unavailable beginning at 9 p.m. ET Thursday, April 21, and will return to service by 7 a.m. ET Friday, April 22, 2016.
This release is focusing on multiple “behind the scenes” updates and fixes.
NOTE: The ability to submit help tickets online will not be available during the downtime listed above.
Apr 14, 2016
eRA Information: Ext-UAT Changes: Do Not Create or Modify Accounts the Week of April 18
Due to an upgrade of the eRA Commons user authentication service on Friday, April 22, 2016, we request that users do not create or modify accounts, including passwords in the Ext-UAT environment for ASSIST, System to System (S2S), Commons Demo, and iEdison during the week of April 18. Any changes made during the week may be lost as result of updates to the user authentication service.
We are sorry for any inconvenience this may cause. Please note that this process will not affect the production environment.
Apr 11, 2016
eRA Information: Ext-UAT and Commons Demo Will be Unavailable Tuesday April 12, 2016
The External User Acceptance Test (Ext-UAT) and the Commons Demo environments are scheduled to be offline Tuesday, April 12, 2016 from 9 a.m. to 6 p.m. in preparation for the spring system-wide software release. As part of our standard practice before each quarterly software release, we update the Ext-UAT and Commons Demo environments with the changes that will take place in production the following week.
These changes will be implemented to production starting on Thursday, April 21, 2016 at 9 p.m. ET.
We are sorry for any inconvenience this may cause. Please note that this process will not affect the production environment.
eRA Information: Highlights Coming to eRA Commons’ xTRACT
xTRACT, accessed via eRA Commons, allows applicants, grantees and assistants to create research training tables for progress reports and institutional training grant applications.
The following features and improvements are scheduled for the Thursday, April 28, 2016 release of xTRACT.
Bold Trainee Names: User will have the ability to “bold” the trainee name within a publication citation, enabling them to comply with the instructions for table 5 (publications). This will be accomplished by using HTML bold tags ( <b>name</b> ) when entering the name of a trainee in the list of authors for a publication.
For example, if the third name (John Q. Public) in this list is the name of the trainee:
Adams, John; Jefferson, Thomas; Public, John Q.; Madison, James; Hancock, John; 1776; The Preamble; Declaration of Independence; Volume 1; pp. 1-15
the user will input the data as:
Adams, John; Jefferson, Thomas; <b>Public, John Q.</b>; Madison, James; Hancock, John; 1776; The Preamble; Declaration of Independence; Volume 1; pp. 1-15
When the training table is rendered as final PDF or in Preview, xTRACT will recognize the HTML syntax and make the name bold. Users are encouraged to update publication text for Table 5 to include this HTML syntax now so that when the release is complete, the tables will be correctly formatted with the desired portion of the citation in bold.
- Editable selection fields: When adding institution names and departments on post-training positions and for prior institutions for Applicants and Entrants, the desired institution or department may not be part of the database to be presented as a selectable option. In these cases, when the user needs to cite these organizations, they will have the ability to type in the name and save the information directly to the field.
- User Interface (UI) improvements: xTRACT is getting updated to the contemporary web style seen in other parts of eRA Commons (see March’s eRA Items of Interest). For example, xTRACT will use type-ahead drop-down lists (also referred to as “predictive search”) in certain places. This means as the user begins to type in the field, the system immediately displays matching results, and thus reducing the time it takes to find the desired result.
Participating Department and Programs Old style where a % was used to initiate a wild card search vs. New where adding characters narrows the search.
Apr 07, 2016
eRA Information: Ext-UAT for ASSIST, S2S Submissions, Commons Demo, and iEdison Will be Unavailable Friday Morning 7-9 AM
The External User Acceptance Test (Ext-UAT) environment for ASSIST, System to System (S2S), Commons Demo, and iEdison will be brought offline from 7 a.m. to 9 a.m. Friday, April 8, 2016.
This is part of the standard testing environment maintenance window as noted at: http://grants.nih.gov/grants/ElectronicReceipt/system_testing.htm
We are sorry for any inconvenience it may cause. Please note that this process will not affect the production environment.
Mar 25, 2016
eRA Update: Issue in xTRACT with Participating Departments/Programs Has Been Resolved
The issue in xTRACT affecting Participating Departments or Programs has been resolved. Please review your census data to ensure that it is complete and accurate.
Original message below sent Friday, March 25, 2016 at 10:44 a.m. ET
eRA Alert: Issue Discovered in xTRACT with Participating Departments/Programs
An issue has been discovered in xTRACT. If a Research Training Dataset (RTD) table is updated and Participating Departments or Programs are removed, the result will be a loss of previously entered census data.
The issue is being addressed and should be resolved shortly. In the meantime, please do not remove any Participating Department or Programs from any RTDs until further notice.
We are sorry for any inconvenience it may cause. Once we have confirmed the issue has been resolved, you will want to check any census data you have entered previously. If you have discovered that you have been affected by this issue, you will need to reenter the missing data.
Mar 24, 2016
eRA Enhancement: New Account Management Feature Planned for March 25 Software Release
eRA will be performing software updates to the Account Management System (AMS) as part of a release scheduled for deployment tomorrow, Friday, March 25, 2016. AMS is used by Signing Officials and other authorized staff to create, manage and search user accounts in eRA Commons.
The release will not require system downtime and is not expected to disrupt any of the eRA systems. Users should have full access to these systems throughout the release.
New Feature in AMS Module
- Gives users the ability to run reports based on user roles via a new tab labeled AMS User Reports.
For details, please see the AMS Release Notes, AMS Online Help and AMS User Guide.
Mar 07, 2016
NIH eRA Items of Interest - March 2016
Prior Approval…

Yes, you read that right. Soon you, Principal Investigators (PIs), will have the power of requesting Prior Approvals electronically! Coming to eRA Commons, PIs will be able to seek prior approval from the granting agency via eRA Commons for submitting applications with direct costs of over $500K or more for any budget year. The request will be initiated and submitted before the submission of the grant application. The PI will need to provide justification for the request, along with a detailed budget that will be uploaded as a 10 page limit PDF.
PIs will also be able to initiate requests electronically to withdraw their applications. These requests will be routed to a Signing Official (SO), who can then submit them. Like the $500K request, they will need to provide a justification for withdrawing the application.
The hope is to add this functionality to eRA Commons by late spring. It is important to remember that the SO can still initiate and submit both types of request.
And wouldn't life be great if we all had this? It would save me the hassle of asking my personal SO (Spouse, Officer-in-charge)… "Hon… can I take the Harley to work?" "Hon… can I watch Star Wars again (for the 317th time)." "Hon… can I take a nap?"
The More Things Change…

At this point you should be asking yourself, "Why is there a picture of Bootstrap Bill from the Pirates of the Caribbean in an article about change?" Well, let me tell you…
You may be noticing some frequent changes, both minor and major, in the way eRA Commons looks and works. In the last year, we have introduced new layouts in such things as the Personal Profile screen; new mobility functionality; updated Federal Financial Report; and Grant re-assignment just to name a few.
And like Bootstrap, these changes have come slowly, little bits at a time, as the different development teams work with various aspects of Commons. Parts of Commons are still looking old, other parts are more updated and some are just plain awesome. The most recent iteration of the look and feel comes from a web framework called Bootstrap (and there it is! The connection to Bill!)
Bootstrap provides an increase in readability with visual friendly spacing; scalability, also called responsive design (this allows information on the screen to adapt to various display sizes, from full size monitors, to tablets, to smart phones); and intuitive functionality.
And like Bootstrap Bill, in the end, everything will look amazing! As each development team tackles the different components of Commons, the more it will all look, feel, and function the same. (Just like Bootstrap Bill, who stays aboard the Flying Dutchman with his son Will, and his skin problem cleans up nicely).
Changes in Security

Speaking of change, we continue to improve the security of important information that you are asked to provide as part of the grant process.
Part of this increased security deals with your personal information: specifically birthday and social security information. After an account is created, the user is encouraged to complete the Personal Profile form for eRA Commons. This includes your birthday and the last four digits of your Social Security Number.
What happens now is that once information is added to the profile form and the form is saved, those fields cannot be edited. If that information needs to be updated, it will be necessary to call the eRA Service Desk to get the proper assistance to make the change.
We encourage people to complete these fields for both account identification reasons and reporting purposes. And in general, everyone should just make sure their profile is up to date for lots of different reasons.
From Blue Crabs to the Windy City: Regionals 2016

Once again, all your favorite NIH folks are hitting the road again!
Our first stop is at Baltimore's Inner Harbor. Optional full and half day workshops will take place on Wednesday, May 11, followed by 2-days of sessions on Thursday and Friday, May 12 & 13.
Space is limited and these workshops and seminars traditionally reach capacity early, so register for Baltimore as soon as you can.
If Baltimore in May doesn't work for you, or maybe you prefer Chicago deep dish pizza over blue crab with Old Bay Seasoning? No worries, join us in the Windy City, Chicago, IL, October 26-28, 2016.
Registration for Chicago is just as easy, so sign up soon!
The agenda is full of great learning and sharing opportunities, and, of course there will be the opportunity to meet with NIH staff for the valuable 1:1 sessions.
See You There!
~ Joe
Mar 03, 2016
eRA Alert: Ext-UAT for ASSIST, S2S Submissions and Commons Demo Will be Unavailable Starting Today at 4 PM
The External User Acceptance Test (Ext-UAT) environment for ASSIST, System to System (S2S) and Commons Demo will be brought offline at 4 p.m. today, Thursday, March 03, 2016. The environment is being brought down to apply a critical software patch and is expected to be back in service by 11 p.m. tonight.
We are sorry for any inconvenience it may cause.
Feb 26, 2016
eRA Update: PDF Generation Error in xTRACT is Now Resolved
The issue in xTRACT that was listing trainee names as faculty names in Training Table 8 has been fixed.
Original message below sent Thursday, February 24, 2016 at 4:18 p.m.
eRA Information: Ext-UAT for ASSIST and S2S Submissions Will be Unavailable Monday, February 29, 2016
The External User Acceptance Test (Ext-UAT) environment for ASSIST and System to System (S2S) is scheduled to be offline Monday, February 29, 2016 from 9 a.m. to 6 p.m. in preparation for Forms D release in March. For more information on form changes please see Guide Notice NOT-OD-16-004.
Test Funding Opportunity Announcements will be posted at https://grants.nih.gov/grants/ElectronicReceipt/files/Forms_D.htm .
We are sorry for any inconvenience this may cause. Please note that this process will not affect the production environments.
Feb 25, 2016
eRA Information: Error in PDF Generation Using xTRACT in eRA Commons
xTRACT is the tool in eRA Commons that allows applicants, grantees and assistants to create research training tables for progress reports and institutional training grant applications.
An issue has been discovered where trainee names are being listed as faculty names in Training Table 8. We hope to have the issue fixed as soon as possible and will provide updated information.
We are sorry for any inconvenience this may cause.
eRA Update: eRA Commons Status Screen Now Available
We have restored access for PIs and ASSTs to the eRA Commons Status Screen. As we reported earlier, PIs can also access the mobile site at: https://m.era.nih.gov/cmb.
(Original message below sent Wednesday, Feb. 24, at 12:56 p.m.)
Feb 24, 2016
eRA Alert: eRA Commons’ Status Screen Unavailable to PIs and ASSTs, SOs Unaffected
eRA Commons’ Status Screen is currently unavailable to Principal Investigators (PIs) and delegated Assistants (ASSTs) through the normal eRA Commons login, however it remains available to the Signing Officials (SOs). Other eRA Commons functions remain operational. The new mobile site for Program Directors/Principal Investigators (PD/PIs) is not affected. PD/PIs can access the mobile site at: https://m.era.nih.gov/cmb
PIs can simply go to the eRA Commons mobile login page and provide their credentials as they normally would when accessing eRA Commons. We hope to have the system returned to full availability soon. We will provide updated information as soon as possible.
We are sorry for any inconvenience this may cause.
Feb 12, 2016
eRA Information: Ext-UAT for ASSIST and S2S Submissions Will be Unavailable Thursday, February 18, 2016
The External User Acceptance Test (Ext-UAT) for ASSIST and System to System (S2S) environments are scheduled to be offline Thursday, February 18, 2016 from 9 a.m. to 6 p.m. in preparation for Forms D release later this winter (March). For more information on form changes please see Guide Notice NOT-OD-16-004.
As part of the transition to Forms D, three of the new forms will be added to the Ext-UAT environment on Thursday. The three forms are:
- PHS 398 Career Development Award Supplemental Form
- PHS 398 Cover Page Supplemental Form
- PHS Inclusion Enrollment Form
Test Funding Opportunity Announcements have been posted at https://grants.nih.gov/grants/ElectronicReceipt/files/Forms_D.htm
We are sorry for any inconvenience this may cause. Please note that this process will not affect the production environments.
Feb 03, 2016
eRA Enhancements: Highlights of eRA Commons' xTRACT Release Scheduled for Thursday, February 4
eRA Commons’ Extramural Trainee Reporting And Career Tracking (xTRACT) system will undergo a maintenance release this Thursday, February 4, 2016. These are just some of the features being rolled out for this release; check out the release notes, following the release, for more information on features and fixes.
NOTE: The maintenance process will not affect other aspects of the production environment for eRA Commons.
New Features
- Subsequent Grants Updated for Trainee or Student When Training End Date is Entered
- When a training end date is entered in the End Date (when Trainee Left Program) field on the Participating Trainee Detail screen for a participating trainee or student, the subsequent grants for that trainee or student are prepopulated from available NIH sources provided that no subsequent grants were previously entered for that trainee or student for that particular Research Training Dataset (RTD).
- Added Feature for Uploading Non-NIH Funding Sources is now Available
- A new feature in xTRACT for uploading non-NIH funding sources is accessed by clicking the Institution Data tab and clicking on the Upload Funding Sources hyperlink.
- Commons User ID now Displaying in Participating Trainees List
- Previously the Commons User ID was not always displaying in the Participating Trainees list for a Research Performance Progress Report’s (RPPR) RTD.
- Non-NIH Sources of Support now Displaying Correctly in Preview PDF
- Previously the non-NIH sources of support were being displayed incorrectly in the PDF when the Preview PDF hyperlink was clicked in xTRACT. These sources of support applied to any non-NIH sources of support under the Summary of Support During Training in the following tables:
- Table 8A – Program Outcomes: Predoctoral
- Table 8C – Program Outcomes: Postdoctoral
- The correct abbreviations listed below now display: NSF Other Fed Univ Fdn Non-US Other
- Previously the non-NIH sources of support were being displayed incorrectly in the PDF when the Preview PDF hyperlink was clicked in xTRACT. These sources of support applied to any non-NIH sources of support under the Summary of Support During Training in the following tables:
- Training Grant Search now Displaying Type 5 Grants Correctly
- Previously when a Type 2 (competing renewal) grant application had been submitted and its status was Pending Review, the previously awarded Type 5 in the grant family was not appearing in the search results on the Search for Training Grants screen. Consequently, if a user wished to start preparing for a resubmission of the competing renewal, there was no way to do so in xTRACT.
- The preceding awarded Type 5 grant is now returned in the search results on the Search for Grants screen.
- OTH Degree Text is now Displaying in the Training Table PDF
- If a faculty member or trainee/student received an OTH (Other) type of degree, it is required to enter what type of degree was earned in the Other Degree Text field on various screens in xTRACT. This Other Degree Text was not being displayed where appropriate in the Training table PDF.
- The Other Degree Text applies to any degrees listed in the following tables:
- Table 8A – Program Outcomes: Predoctoral
- Sections: Terminal Degree(s) received and Year(s)
- Table 8C – Program Outcomes: Postdoctoral
- Sections: Doctoral Degree(s) and Year(s) or Degree(s) resulting from Postdoctoral training and Year(s)
- Table 8A – Program Outcomes: Predoctoral
- Doctoral Degrees now Appearing in Training Table 8C
- Previously doctoral degrees were not appearing in the column labeled Doctoral Degree(s) and Year(s) in Table 8C whenever the doctoral degree earned date and the postdoctoral trainee’s entry program date were in the same month and year.
- When Performing Training Grant Searches Assistants can now See all of their PIs
- When performing training grant searches Assistants were not always seeing all of their Principal Investigators (PIs) as expected in their Delegator List in xTRACT.
- Subsequent Grants Updated for Trainee or Student When Training End Date is Entered
Jan 22, 2016
eRA Update: URL for PI Mobile Friendly Status Screen Is Now Available
The technical issue that was making the PI mobile friendly status screen unavailable was addressed. Most Recent message sent Wednesday, January 20, 2016, 11:43 a.m. ET
Jan 20, 2016
eRA Alert: URL for PI Mobile Friendly Status Screen Unavailable
A technical issue is currently making the URL for the new mobile friendly PI Status Screen unavailable. We are working diligently to address this issue and restore service. We will provide more information once it becomes available. We are sorry for any inconvenience this may cause.
Original message sent Wednesday, January 13, 2016, 4:11 p.m. ET
Jan 19, 2016
eRA Enhancements: Highlights of eRA Commons’ Upcoming Release & Scheduled Downtime for Thursday, January 21
Scheduled Downtime for January Release:
- eRA Commons and ASSIST will be unavailable beginning at 9 p.m. ET Thursday, January 21, and will return to service by 7 a.m. ET Friday, January 22, 2016. When the system is available again, you will see new features and functionality have been added to the system. The following are highlights of eRA Commons’ upcoming January software release. These are just some of the many features being rolled out; check out the release notes, following the release, for more information on features and fixes.
- Highlights of eRA Commons’ January Software Release
- Updates in eRA Commons
- NIH has added several new features to eRA Commons:
- Principal Investigator (PI) Screens Redesigned for Status, JIT, and Closeout
- The eRA Commons’ Status screen for PIs has been redesigned with a refined interface, a responsive design and grouping of information that is user friendly. Searches on the Recent/Pending eSubmissions and List of Applications/Grants screens will now provide a total count of applications/grants at the top of the screen. The results will be grouped by Application ID/Grant number and will be expandable and collapsible as needed.
- Additionally, eRA has updated the look of both the Just in Time (JIT) and Closeout screens for PIs.
- Both have been reconfigured to improve readability and flow.
- Both now support “Drag & Drop” functionality to upload files while dynamically validating file size and format.
NOTE: Signing Officials will see this same functionality once the redesign of the SO Status Screen is completed later this year.
Also a condensed version of the Status screen for PIs is now available for use on mobile devices through a special URL — https://m.era.nih.gov/cmb. See eRA News for more information on this topic.
- Electronic Signature of New Organization Registration in eRA Commons
- The registration process for eRA Commons will be made less burdensome: Signing Officials will now be able to sign electronically instead of signing a hard copy and faxing the registration form to eRA.
- New Functionality for Delegates
- Delegates will benefit from new functionality within eRA Commons. If Status is delegated to an person from multiple PIs, the selection of the person for whom they are acting will remain throughout their connection to Commons unless they specifically change the Status setting.
Updates in ASSIST
Because of the upgrade in December 2015, there are no significant changes to ASSIST in this release. However, we would like to remind you of the powerful features that were added in that upgrade.
- Copy Application with Attachments
- The Copy application feature now includes attachments! When copying an application, you can choose to include all the attachments from the original version.
NOTE: When copying an application with attachments, ASSIST will show the status of the application as “Incomplete” until the copied version is saved. Be sure to click the Save button to update the status.
- Transition of ASSIST Complete
- ASSIST is now an option for:
- all single and multi-project, competing grant applications;
- single-project administrative supplements;
- single-project, post-award successor-in-interest (type 6) requests; and
- single-project, post-award change of institution (type 7) requests.
- ASSIST is now an option for:
NOTE: The ability to submit help tickets online will not be available during the downtime listed above.
Jan 14, 2016
eRA Information: eRA Commons and ASSIST Will Be Unavailable Saturday, January 16, 2016
eRA Commons and ASSIST will undergo maintenance procedures and are scheduled to be unavailable Saturday, January 16, from 10 p.m. to midnight. We hope to have the systems up as soon as possible.
We are sorry for any inconvenience this may cause. If you feel this procedure has impeded your ability to submit an on time application, be sure to follow the Guidelines for Applicants Experiencing System Issues.
Jan 13, 2016
eRA Information: eRA Commons Status Screen for PIs Now Mobile Friendly
Beginning on January 15, 2016, a new URL will be available to PIs for mobile access to their status information in eRA Commons. The new URL will be:
This new mobile access means it will be significantly easier for PIs to track and manage grant applications and awards because the Status screen will be easily viewable on a range of devices such as tablets and smartphones. PIs can simply go to the eRA Commons mobile login page and provide their credentials as they normally would when accessing eRA Commons.
The mobile site is designed to provide the basic and necessary information PIs need to track their application submissions and awards. The status screen, resizable due to responsive design, provides a table of all their applications. The applications are grouped based on status, going from Received, Awarded, Pending, Withdrawn, to Not Funded. The column headings are Project Number, Status, Project Title and Date.
Each application can be expanded to show important information such as Priority Score, Percentile, Scientific Review Group (SGR) information and links to other resources, such as the application image and/or summary statement. There is also a Contact section that provides access to a PI’s assigned Scientific Review Officer (SRO), Grants Management Specialist (GMS) and Program Official (PO).
Additionally, at the top of the screen is a large search/filter field. When a PI simply starts to type in any information from any of the columns, the results will be dynamically updated as they type.
Jan 11, 2016
eRA Information: Ext-UAT and Commons Demo Will be Unavailable Tuesday January 12, 2016
The External User Acceptance Test (Ext-UAT) and the Commons Demo environments are scheduled to be offline Tuesday, January 12, 2016 from 9 a.m. to 6 p.m. in preparation for the winter system-wide software release starting on Thursday, January 21, 2016 at 9 p.m. ET. As part of our standard practice before each quarterly software release, we update the Ext-UAT and Commons Demo environments with the changes that will take place in production the following week.
We are sorry for any inconvenience this may cause. Please note that this process will not affect the production environment.




 eRA Intranet
eRA Intranet