Closeout—Final Research Performance Progress Report for NIH Awards
Agency-Specific Instructions: NIH ONLY. The policy guidance in this topic applies to NIH only. See Closeout—Final Research Performance Progress Report for NON-NIH Awardsfor Final RPPR guidance for awards that are NOT funded by NIH.
As of January 1, 2017, a Final Research Performance Progress Report (Final RPPR) is required for any grant that has ended and any grant that is not to be extended through award of a new competitive segment. The report is due within 120 days of the end of the project period. This report should be prepared in accordance with instructions provided by the awarding component. See NIH Implementation of Final Research Performance Progress Reports (Final RPPR) — Guide Notice NOT-OD-17-022.
Effective February 9, 2017, if the recipient organization has submitted a renewal application on or before the date by which a Final Research Performance Progress Report would be required for the current competitive segment, then submission of an Interim RPPR via eRA Commons is required. The Interim RPPR (IRPPR) will be used for the submission of a Competing Renewal application (Type 2). See NIH Implementation of the Interim RPPR while a Renewal Application is Under Consideration — (Guide Notice NOT-OD-17-037).
Both the Interim RPPR and the Final RPPR are identical in process and information required. The difference between them is when and where they are made available to initiate and submit. The Interim RPPR link is available to the signing official (SO) and principal investigator on the Status screen when a grant is eligible for submission for a Competing Renewal application.
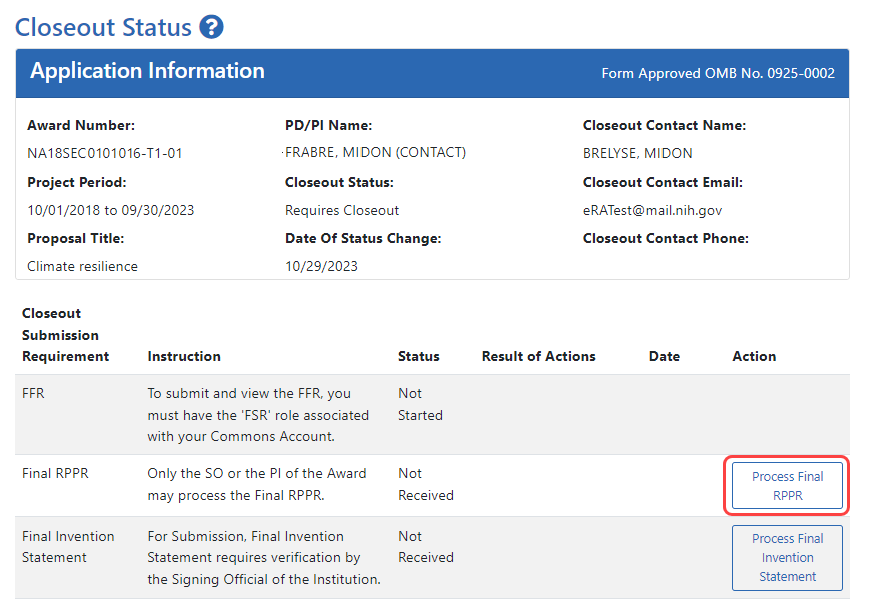
The Final RPPR is only available as part of the Closeout process and the Process Final RPPR link only appears on the Closeout Status screen.
The format of the Interim RPPR, the Final RPPR, and the annual RPPR are the same, making it easier for grantees to navigate and complete them.
Differences between Interim/Final RPPR and the annual RPPR are:
- In the Interim/Final RPPR, only D.1 is required in the Participants section
- Sections F: Changes and Section H: Budget are not part of the Interim/Final RPPR
- Section I: Outcomes is required for both the Interim/Final RPPR
Note about Interim RPPR and Final RPPR
- The Interim RPPR (IRPPR) is used when you are submitting a Competing Renewal application (Type 2). If you opt NOT to apply for a Competing Renewal, complete the Final RPPR as you normally would within 120 days of the project end date. If you are going to complete a Competing Renewal application (or have already submitted such an application), you will submit an Interim RPPR. This must be submitted within 120 days of the project end date.
- If you are awarded the renewal, the Interim RPPR will be treated as your annual RPPR and no other progress reporting will be needed for that segment of the study. If the application is NOT awarded, then the Interim RPPR will be accepted as the Final RPPR.
Submit Your Final RPPR
- Log into eRA Commons as the contact PD/PI for an award, and access the Closeout Status screen. To access the Closeout Status screen, see Accessing the Closeout Screen (SO and PI).
- Click the Process Final RPPR button in Closeout Status.
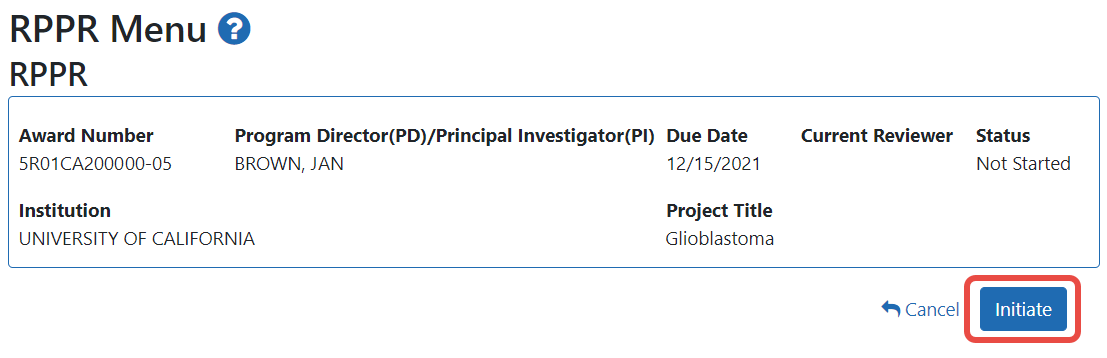
The RPPR Menu screen appears.
If the Final RPPR has already been initiated, then no Initiate button appears and you should skip the next step.
-
If an Initiate button appears on this screen, click the Initiate button to start the Final RPPR.
NOTE: Only the PI can initiate the RPPR, and only the SO can submit it.
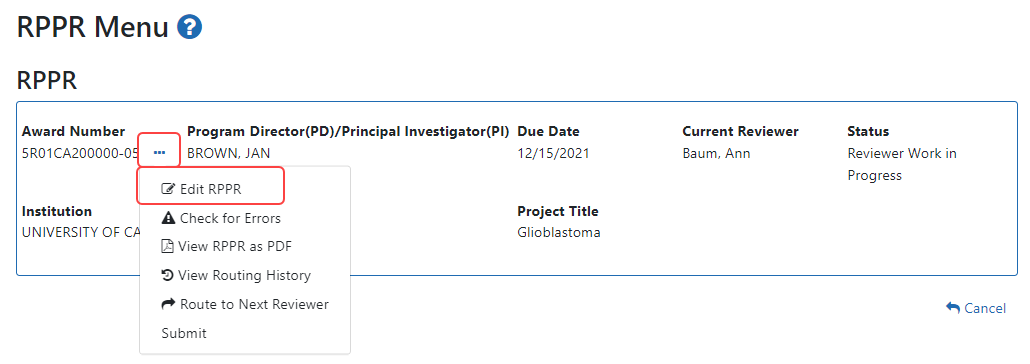
-
Click the Initiate button to create the Final RPPR. The Final RPPR Menu will then change, showing the option to Edit the Final RPPR under the thee-dot ellipsis menu:
-
Select Edit RPPR from the three-dot ellipsis menu, fill out the RPPR, and Submit it (only SOs can submit). For more details on filling out the RPPR, see Editing the RPPR Forms.
Field-by-field guidance is available for completing the NIH, AHRQ, and VA Research Performance Progress Reports (RPPRs). Refer to the NIH and Other PHS Agency RPPR Instruction Guide (https://grants.nih.gov/grants/rppr/rppr_instruction_guide.pdf) for completing Sections A-I of the RPPR. This guide does not apply to Department of Commerce (DOC) awards.
