Closeout—Final Invention Statement
Either a program directory/principal investigator (PD/PI) or a signing official (SO) can edit a final invention statement, but only an SO can submit it to the agency. Use this procedure to complete a Final Invention Statement that lists inventions that were conceived as part of the research grant.
Reporting Inventions on the Final Invention Statement
Reporting No Inventions on the Final Invention Statement
NOTE: Only a signing official (SO) or a user with delegated report submit authority can submit the reports or other data; the program director/principal investigator (PD/PI) can upload, preview, delete and save the closeout report submission package, but cannot complete the final step to submit.
NIH and AHRQ Final Invention Statement Policy Guidance
Agency-Specific Instructions: NIH and AHRQ only. The following policy guidance applies to NIH and AHRQ-funded awards only.
You must submit a Final Invention Statement (FIS) within 120 days following the termination of a grant award. The statement should include all inventions that were conceived or first reduced to practice during the course of work under the grant or award, from the original effective date of support through the date of completion or termination.
Policy: For policy information on awards funded by the Department of Health and Human Services, refer to the Procedure for Submission of Final Invention Statement and Certification.
If your award is funded by a non-NIH or non-AHRQ source, check with your awarding agency for policy guidance on final invention statements.
Reporting Inventions on the Final Invention Statement
To complete a final invention statement when inventions need to be reported:
- Log in to eRA Commons as an SO or a PI and access the Closeout Status screen. To access the Closeout Status screen, see Accessing the Closeout Screen (SO and PI).
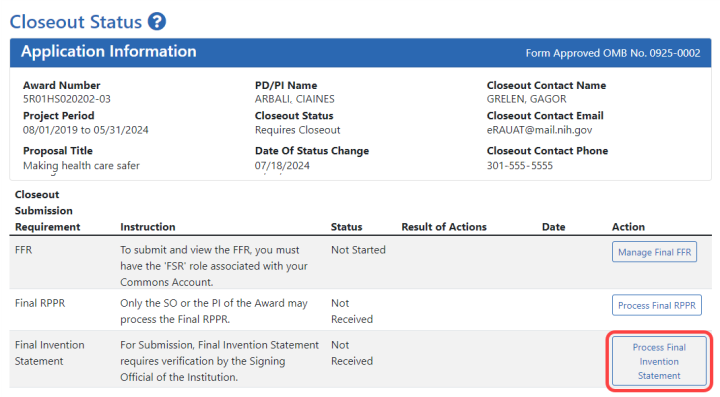
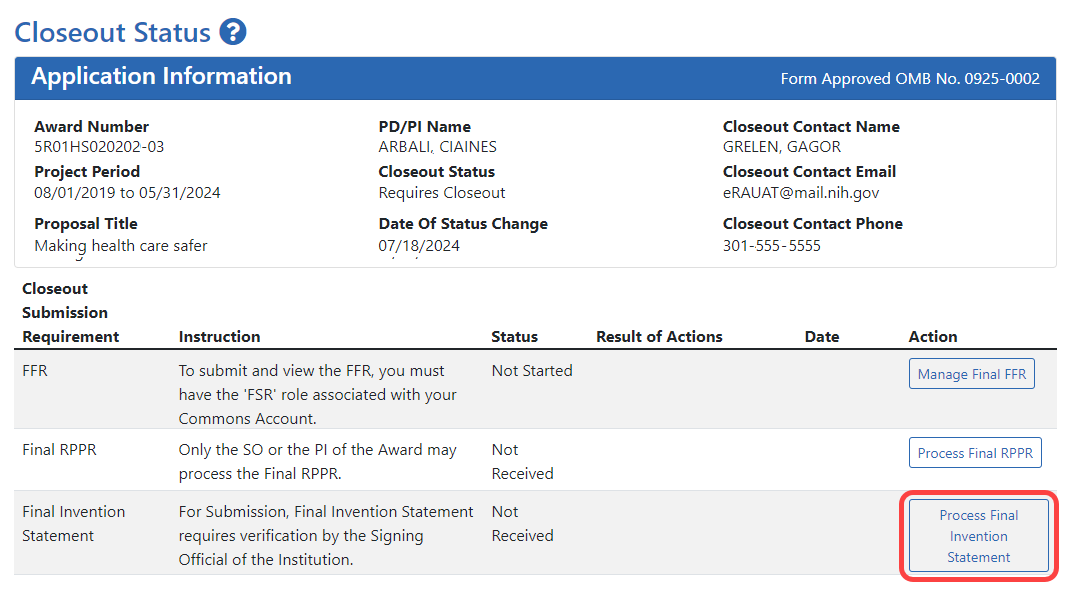
![]() The Closeout Status screen appears.
The Closeout Status screen appears.
![]() Below is a sample Closeout Status screen showing the task. Depending on the awarding agency, different tasks might appear than those shown.
Below is a sample Closeout Status screen showing the task. Depending on the awarding agency, different tasks might appear than those shown.
- Click the Process Final Invention Statement button on Closeout Status.
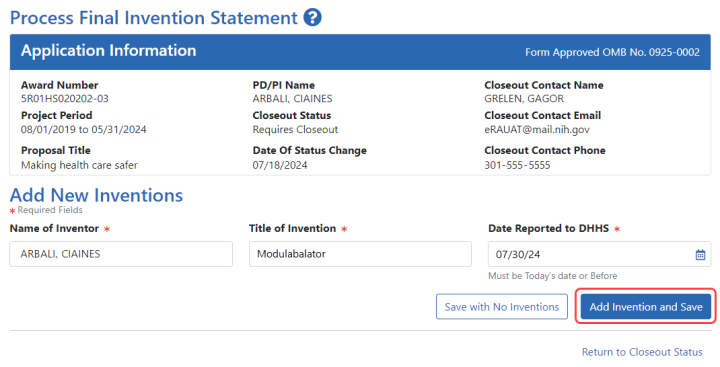
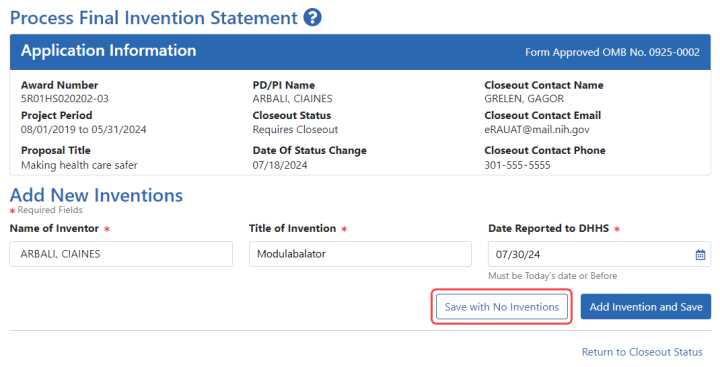
![]() The Process Final Invention Statement screen displays.
The Process Final Invention Statement screen displays.
-
To add an invention:
| a. | Type the Name of Inventor, Title of Invention, and the Date Reported to DHSS (the date you want to officially report it to the Department of Health and Human Services). |
| b. | Click the Add Invention and Save button. |
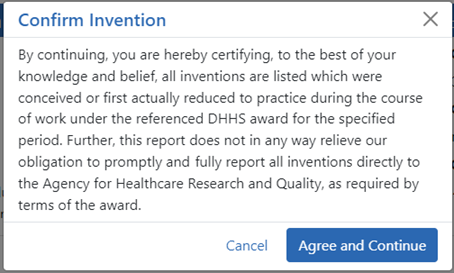
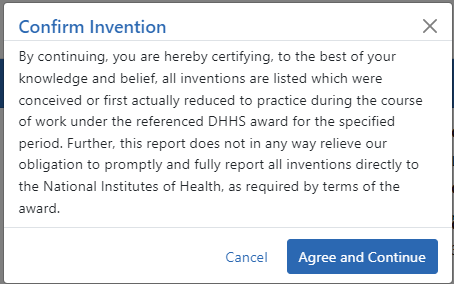
The Confirm Invention popup appears with customized language depending on whether the award is funded by ![]() AHRQ or
AHRQ or ![]() NIH.
NIH.
| c. | Click Agree and Continue in the popup even if you have more inventions to add. |
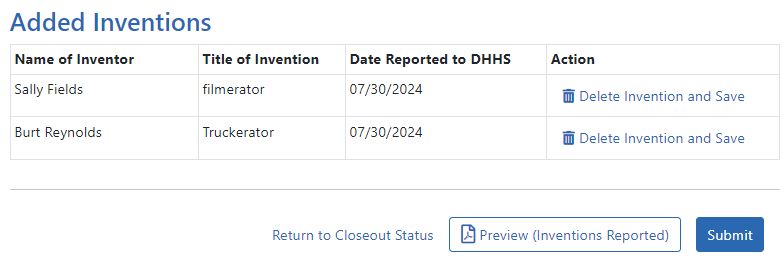
The invention is now listed under Added Inventions at the bottom of the screen. As long as the final invention statement has not yet been submitted, either a PI or SO who views this screen can click an item's Delete Invention and Save link in the Action column to remove it.
-
To add additional inventions, repeat the previous step as many times as necessary. Any inventions you define are saved immediately.
NOTE: Click the Preview (Inventions Reported) button to download a PDF copy of the final invention statement form filled out with the entered information and grant information.
-
When all the inventions are listed at the bottom of the screen, click the Submit button to submit the invention statement to the awarding agency. (Only the SO can submit—the PI does not see a Submit button.)
The Confirm Invention popup appears again.
-
Click Agree and Continue again to complete submission.
You are returned to the Closeout Status screen and the Final Invention Statement task now indicates the Status of Submitted and shows submission details.
After final invention statement submission, the PDF form that was submitted is available on the Closeout Status screen by clicking the View button next to the Final Invention Statement task.
Reporting No Inventions on the Final Invention Statement
To complete a final invention statement certifying that no inventions exist:
- Log in to eRA Commons as an SO or a PI and access the Closeout Status screen. To access the Closeout Status screen, see Accessing the Closeout Screen (SO and PI).
The![]() Closeout Status screen appears.
Closeout Status screen appears.
![]() Below is
Below is ![]() a sample Closeout Status screen showing the task. Depending on the awarding agency, different tasks might appear than those shown.
a sample Closeout Status screen showing the task. Depending on the awarding agency, different tasks might appear than those shown.
- Click the Process Final Invention Statement button on Closeout Status screen.
![]() The Process Final Invention Statement screen displays.
The Process Final Invention Statement screen displays.
-
Click the Save with No Inventions button.
A success message appears on the top right side of the eRA screen heading. You will also notice that the Preview button is now named Preview (No Inventions Reported).

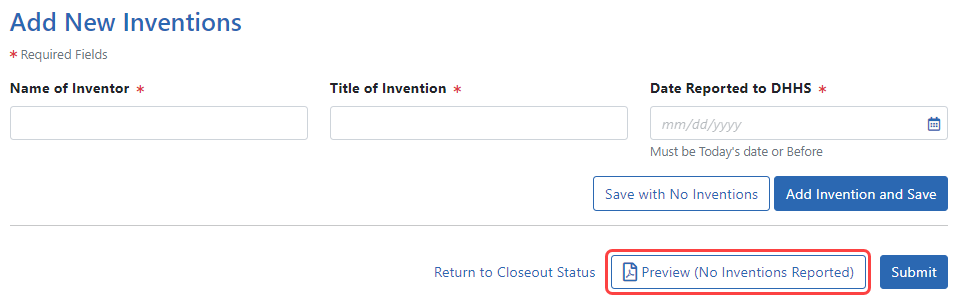
NOTE: Click the Preview (No Inventions Reported) button to download a PDF copy of the final invention statement form filled out with the entered information and grant information.
-
Once you confirm that the Preview button is now renamed to indicate "No Inventions Reported", click the Submit button to submit a statement indicating no inventions are being reported. (Only the SO sees a Submit button and has the ability to submit.)
A certification popup appears.
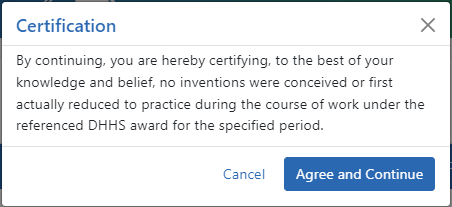
-
Click Agree and Continue in the Certification popup.
You are returned to the Closeout Status screen and the Final Invention Statement task now indicates the Status of Submitted and shows submission details.
After final invention statement submission, the PDF form that was submitted is available on the Closeout Status screen by clicking the View button next to the Final Invention Statement task.