Prior Approval - Other Request
The Other Request type is used in different ways depending on the agency providing the award. Prior Approval "Other Request" can be used for both FDA and NIH awards. The Other Request type appears only for signing officials (SOs) and does not appear for principal investigators (PIs).
For FDA awards, "Other Request" provides a generic request type for signing officials (SO) to make requests for FDA grants. All FDA grants are eligible for the Other Request type.
For NIH awards, "Other Request" is the required method for for signing officials to submit prior approval requests for changes to an approved Data Management and Sharing (DMS) Plan. For NIH awards, the "Other Request" type should ONLY be used for revised DMS Plan prior approval requests. See Special Instructions for DMS Plan Revision Requests.. below.
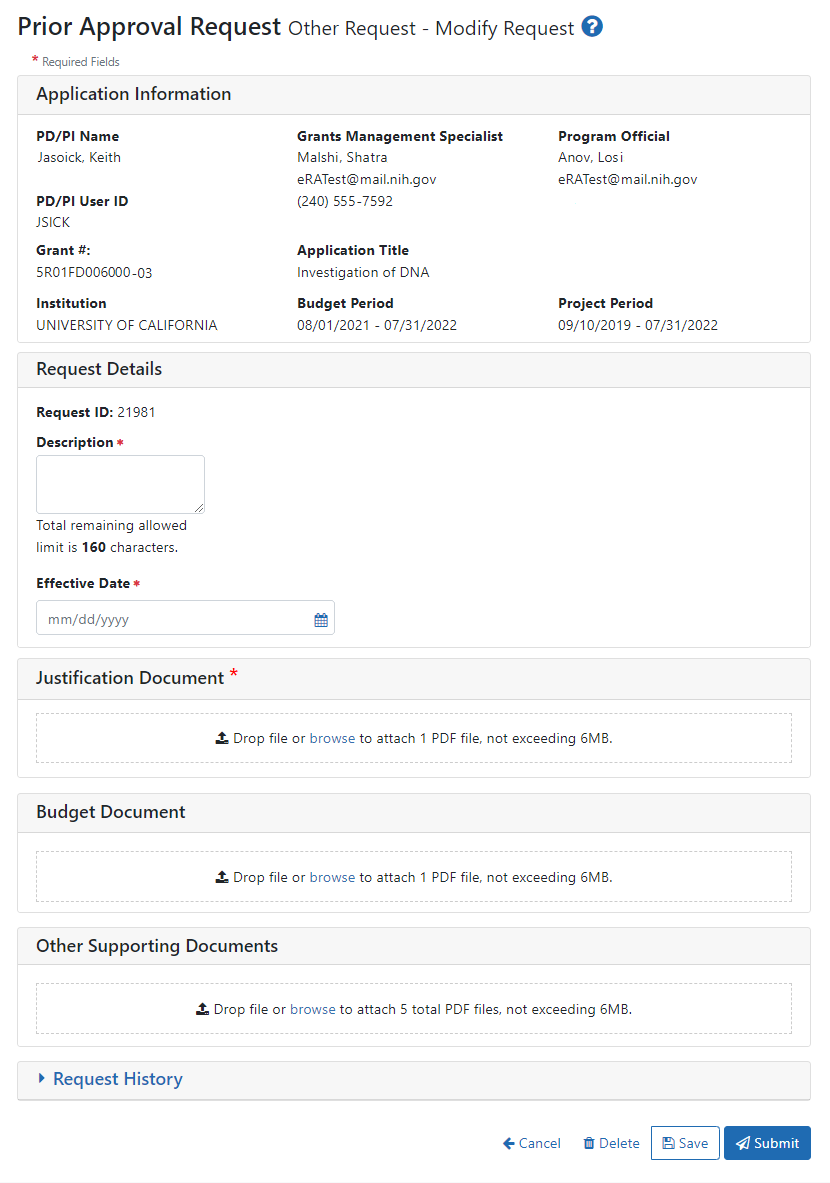
See Viewing and Initiating Requests for details on initiating a request. After initiating an Other Request, you see the following details screen:
-
Must be an FDA or NIH award
-
Must be awarded
-
Must be within the current budget period
-
Grant must not be closed or terminated.
The Other Request can be initiated for any application type matching the above criteria.
-
Fill out all required fields (which have a red asterisk). You can drag and drop a PDF file from your file system onto the Drop file area, or click Browse to locate and select a file.
-
To view or remove a file you have uploaded, click the three-dot ellipsis menu and select View or Delete. If you upload the wrong file, you must delete it in order to upload another, as only one file is allowed to be uploaded.
All uploaded documents must be in PDF format.
Signing officials requesting DMS Plan changes for NIH awards should fill out the form and submit documents related to the change as described below:
Description. Enter “DMS Plan Revision” (without quotations).
Effective Date. Enter the effective date of the requested changes.
Justification Document. Provide the rationale and justification for the requested changes.
Budget Document. Provide if the revised DMS Plan impacts the budget. Include information for current and future budget periods. Note: This is not a supplement request.
Other Supporting Documents. Attach the revised DMS Plan.
Submitting the Request to the Agency
