Export Human Subjects Data to an XML File
Use this process to create an XML file on your local disk that contains a copy of the study's Human Subjects Study (HSS) data.
NOTE: If you are the ClinicalTrials.gov "responsible party" user, you have the option to upload your study's HSS data directly, without having to create an XML file yourself. To do so, follow the instructions at Upload XML Data to ClinicalTrials.gov. Note that the PRS account is granted to an organization by ClinicalTrials.gov. Each organization decides who can use the account to upload studies to CTG. Thus, the “responsible party” could be the signing official or some other official from the organization. If necessary, apply for a PRS account and then proceed with the steps below.
TIP: If you are the ClinicalTrials.gov "responsible party" user, you have the option to upload your study's HSS data directly, without having to create an XML file yourself. To do so, follow the instructions at Upload Study Data Directly to ClinicalTrials.gov.
- Log in to eRA Commons and identify the application/award containing the study record. Click the Human Subjects link
 to go to ASSIST.
to go to ASSIST.
-
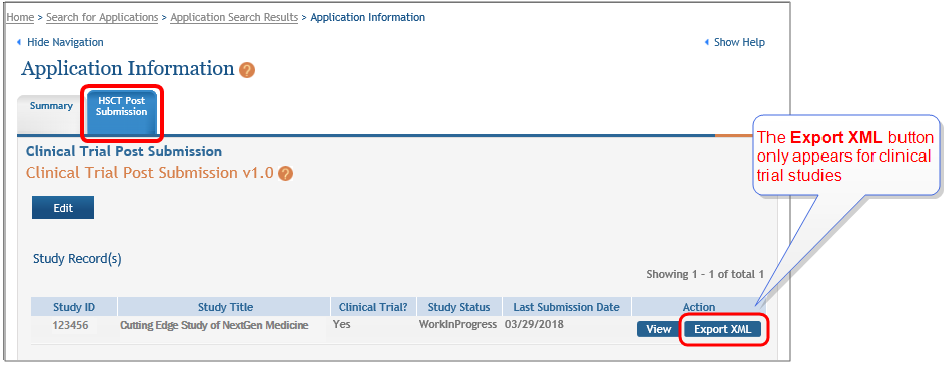
Click the Export XML button to display the
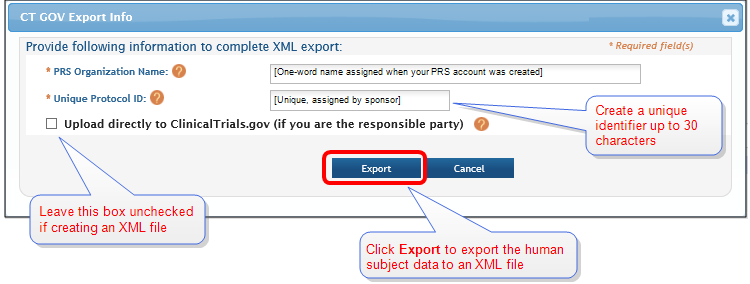
 CT Gov Export Info window.
CT Gov Export Info window.NOTE: The Export XML button is displayed only for Clinical Trial Studies, which are studies that have "yes" responses to all four of questions 1.4a through 1.4d on the Study Record.
- Complete the Organization Name and the Unique Protocol ID Number fields, and then click the Export button. (Leave the Upload check box blank)
NOTE: The Organization Name is the one-word name assigned when your Protocol Registration and Results System (PRS) account was created. The Unique Protocol Identification Number can be any unique identifier that you chose up to 30 characters in length.
The XML file is saved to your computer's default download folder.