Upload Human Subjects Data Directly to ClinicalTrials.gov
NIH applicants/awardees can upload study record data available in the Human Subjects System (HSS) directly to ClinicalTrials.gov, thereby avoiding the need to re-key those clinical study fields at that site.
NOTE: In order to upload study data to ClinicalTrials.gov, the study's sponsoring organization must first have a Protocol Registration and Results System (PRS) account. If necessary, follow the steps to apply for a PRS account.
| 1. | Log in to eRA Commons and identify the application/award containing the study record, and then click the Human Subjects link |
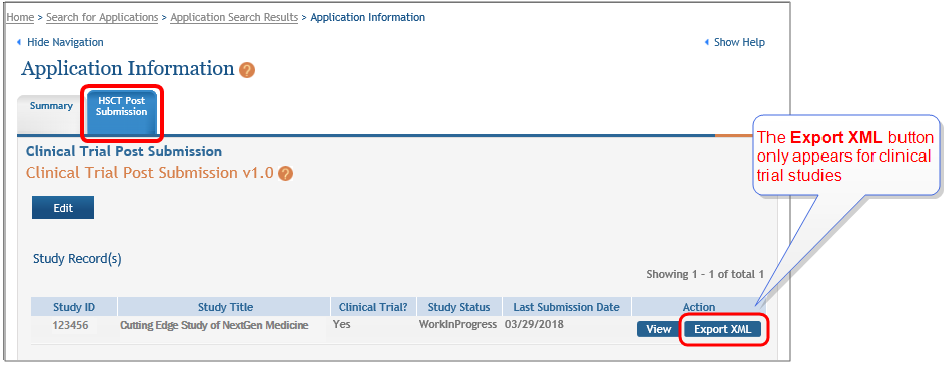
NOTE: The Export XML appears only for study records that propose a clinical trial where the user has entered "yes" responses to questions 1.4a through 1.4d on the Study Record.
| 2. | Click the Export XML button. |
IMPORTANT: The Upload check box only appears for clinical trial study records that have a study ID, but that do not have an NCT# (the ClinicalTrials.gov identifier), and that have not been uploaded previously to ClinicalTrials.gov.
| 3. | Check |
NOTE: The Organization Name is the one-word name assigned when your Protocol Registration and Results System (PRS) account was created. The Unique Protocol ID is a unique identifier up to 30 characters that you chose.
| 4. | Enter the username and password for PRS, and then click the Upload to ClinicalTrials.gov button. Note that ClinicalTrials.gov requires additional information to register a trial. This information, as well as any edits must be done in the PRS system. |
If your upload was successful, the following message displays:
You have successfully uploaded your protocol <protocol ID>. To complete the registration of your clinical trial, you must log into the Protocol Registration and Results System (PRS) at ClinicalTrials.gov (https://register.clinicaltrials.gov/) and complete the required information.
If your upload was unsuccessful, the following message displays:
The ClinicalTrials.gov upload capability for this study is disabled because an NCT number is already associated with the study record or the study record has already been uploaded to ClinicalTrials.gov. If you need to update the ClinicalTrials.gov information for this study, you need to login into the Protocol Registration and Results System (PRS) at ClinicalTrials.gov.
Check Uploaded Data on ClinicalTrials.gov
| 1. | Log into ClinicalTrials.gov PRS with your assigned Organization, Username, and Password.
|
| 2. | Review the uploaded data and add or modify as necessary. |
TIP: For detailed instructions on how to view and modify clinical data on ClinicalTrials.gov, refer to the PRS help system's page on Modifying a Record in PRS.