PHS Inclusion Enrollment
IMPORTANT: The PHS Inclusion Enrollment Report will be discontinued with the rollout of Forms E Applications with due dates beginning in January of 2018. The collection of this data for Forms E applications is moved to the new PHS Human Subjects and Clinical Trials Information Form.
For assistance with the information required on this form, please refer to the appropriate application guide on the How to Apply page.
The PHS Inclusion Enrollment Report form is used for all applications involving NIH-defined clinical research. This form is used to report both planned and cumulative (or actual) enrollment, and describes the sex/gender, race, and ethnicity of the study participants.
For guidance on completing the form in ASSIST, refer to the steps below.
Adding a PHS Inclusion Enrollment Report
To add an Inclusion Enrollment Report:
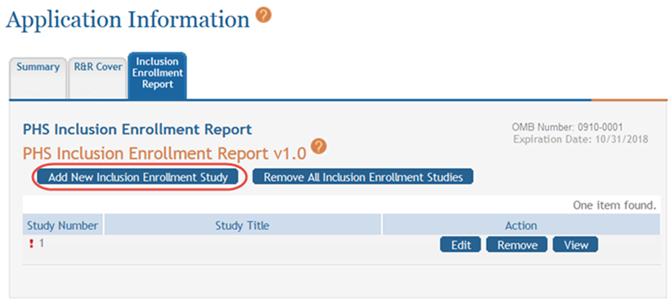
 Select the Add New Inclusion Enrollment Study button. If this is the first report you are adding, you can also select the Edit button for Study 1 located in the Action column of the displayed table.
Select the Add New Inclusion Enrollment Study button. If this is the first report you are adding, you can also select the Edit button for Study 1 located in the Action column of the displayed table.
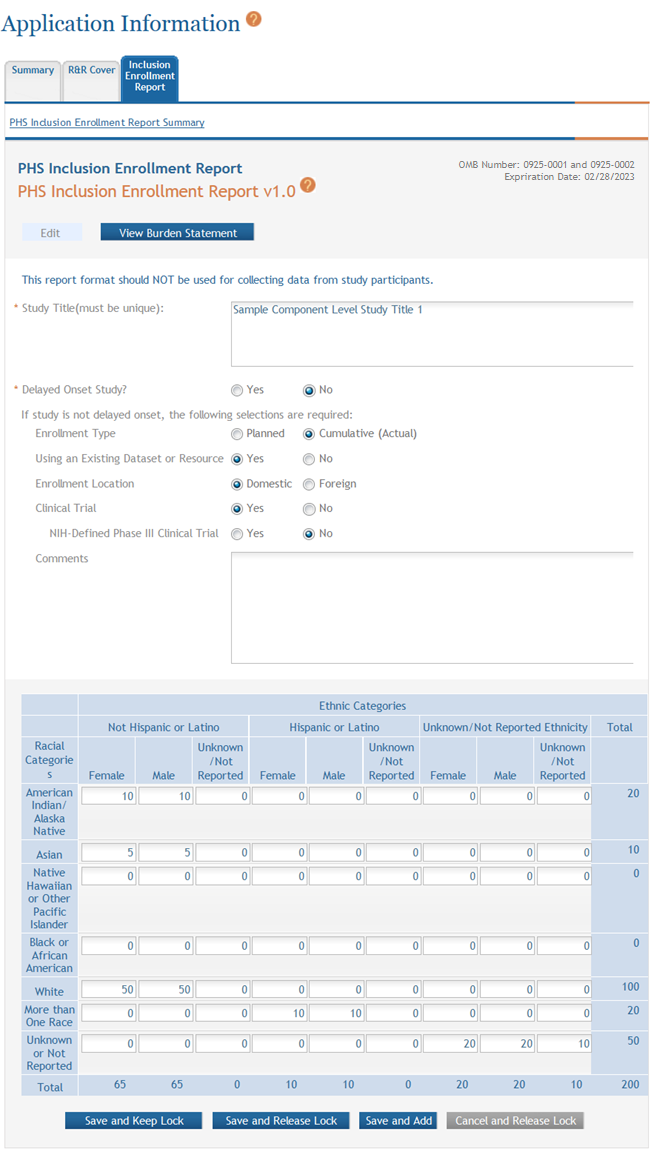
- Complete the required fields and any other appropriate information. Required fields are marked with an asterisk (*).
- Select one of the save options at the bottom of the form to save the data:
- To save the information and keep the form open for further editing, select the Save and Keep Lock options.
- To save the information and close the form, select the Save and Release Lock button.
- To save the data on the current page of the form and to display a new page for entry, select the Save and Add button.
NOTE: Selecting the Cancel and Release Lock button - followed by the Continue button on the confirmation - returns the form to read-only and does not save any of the entered information onto the form.
Viewing and Editing the Inclusion Enrollment Report
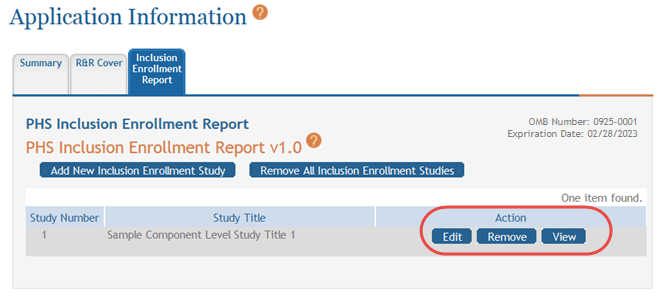
To view and/or edit a cumulative inclusion enrollment study:
To view the information:
- Select the View button to see the form in read-only.
NOTE: Note that once within the read-only form, you can select the Edit button at the top of the form to enter edit mode.
To edit the information:
- Select the Edit button for the study.
- Make your changes.
- Select one of the save options at the bottom of the form to save the data:
- To save the information and keep the form open for further editing, select the Save and Keep Lock options.
- To save the information and close the form, select the Save and Release Lock button.
- To save the data on the current page of the form and to display a new page for entry, select the Save and Add button.
NOTE: Selecting the Cancel and Release Lock button - followed by the Continue button on the confirmation - returns the form to read-only and does not save any of the entered information onto the form.
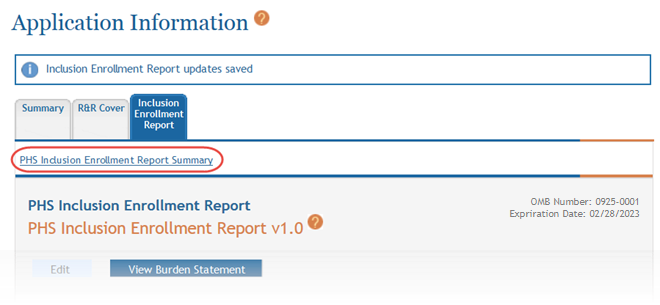
 Select the PHS Inclusion Enrollment Report Summary link to return to the table of enrollment studies.
Select the PHS Inclusion Enrollment Report Summary link to return to the table of enrollment studies.
- Click the PHS Inclusion Enrollment Report Summary link to return.
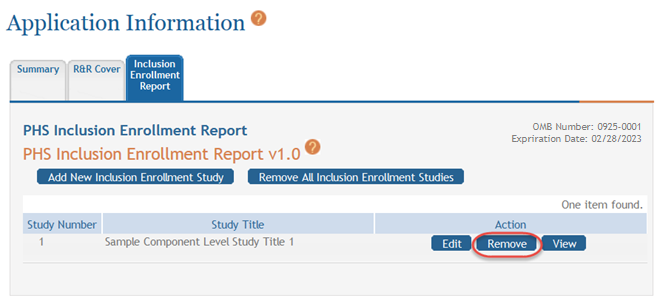
Removing Individual Cumulative Inclusion Enrollment Study
You can remove the studies individually using the buttons found in the Action column of the summary table. To remove an individual study:
 Select the Remove button for the specific study.
Select the Remove button for the specific study.
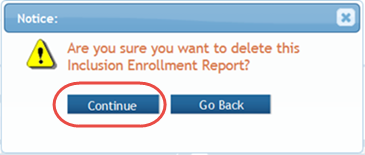
 A confirmation message pops up prompting you to continue or cancel this action.
A confirmation message pops up prompting you to continue or cancel this action.
- Select the Continue button to continue removing the study.
- If you wish to cancel, select the Go Back button or click the X.
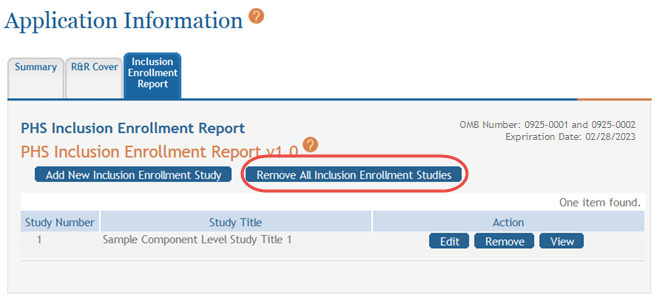
Removing All Cumulative Inclusion Enrollment Studies
You can remove all studies at the same time rather than removing each individual study. Removing all studies also removes the entire form from the component.
To remove all studies:
 Select the Remove All Cumulative Inclusion Studies button at the top of the form.
Select the Remove All Cumulative Inclusion Studies button at the top of the form.
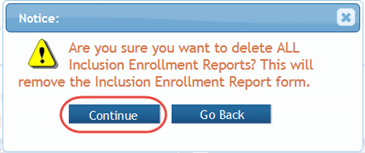
 A confirmation message pops up prompting you to continue or cancel the action.
A confirmation message pops up prompting you to continue or cancel the action.
- Select the
 Continue button to remove all forms.
Continue button to remove all forms.
If you wish to cancel, select the Go Back button