Editing Studies
In order to edit study information, the principal investigators (PIs) or signing officials (SOs) can access the HSCT form using the Human Subjects links in either the RPPR or through the Status screen in eRA Commons. Refer to Access Human Subjects System (HSS) for details.
Human subjects information might need to be updated in the following scenarios:
- Post-award for updates to the Research Performance Progress Report (RPPR), including updates to inclusion enrollment reports and the Clinical Trial Milestone Plan (Section 6)
- Pre-award (post review) for Just-in-Time (JIT) information or correction of human subjects data
- Off-cycle updates as required in the Funding Opportunity Announcement (FOA) or terms and conditions of award
To edit an existing study, log into eRA Commons and access the Human Subjects link via the RPPR or Status tabs.
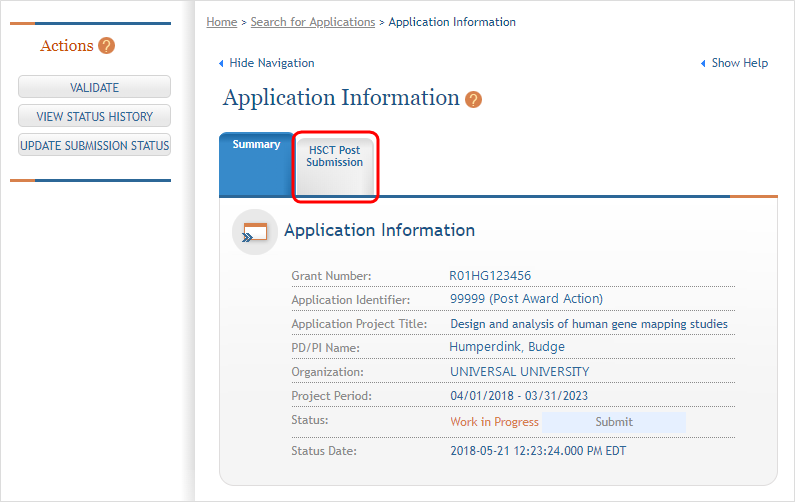
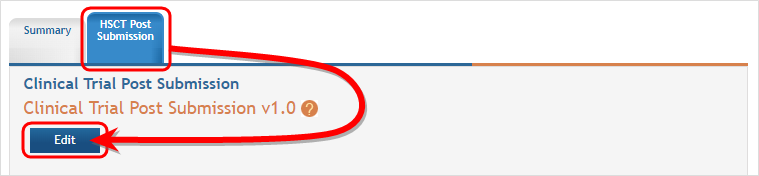
The Application Information screen is displayed, showing a summary of your grant. You have two ways of accessing and editing the study data. Both begin by accessing the HSCT Post Submission tab.
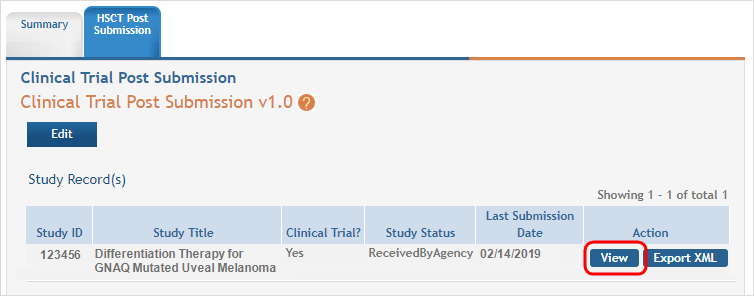
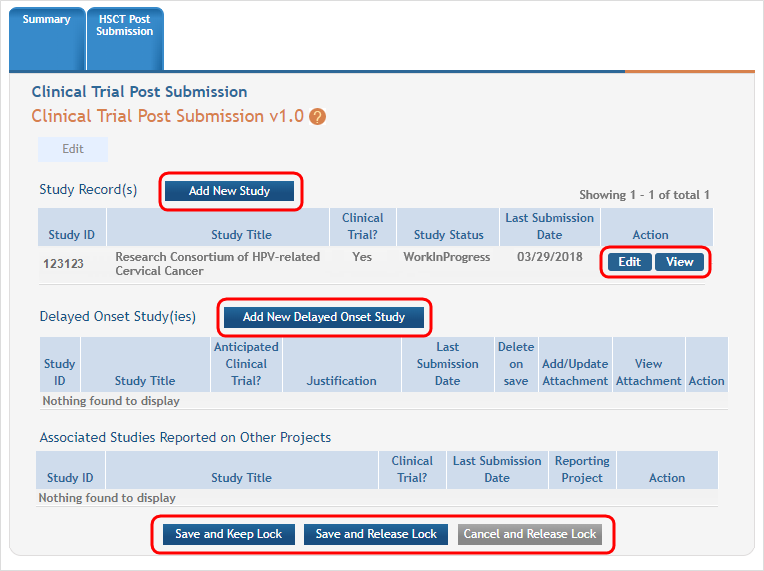
Click on the Human Subjects Post Submission tab. This takes you to a Study Record(s) screen where all study records and delayed onset studies associated with your grant are displayed. ![]() (click to view)
(click to view)
Note: In order to edit, the HSS record must be in Work in Progress status. See How To Change the Application Status and Resubmit for instructions on updating the status.
Option 1
- Click on the View button to open the study record data.
 (click to view)
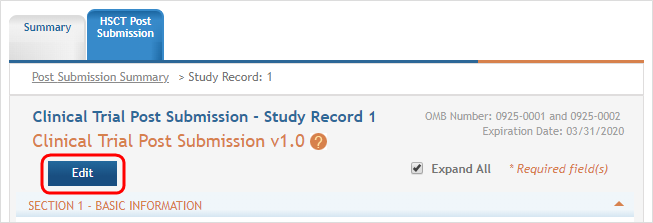
(click to view) - To update the human subjects information on that study, including inclusion enrollment data, click the
 Edit button at the top of the screen.
Edit button at the top of the screen. - The
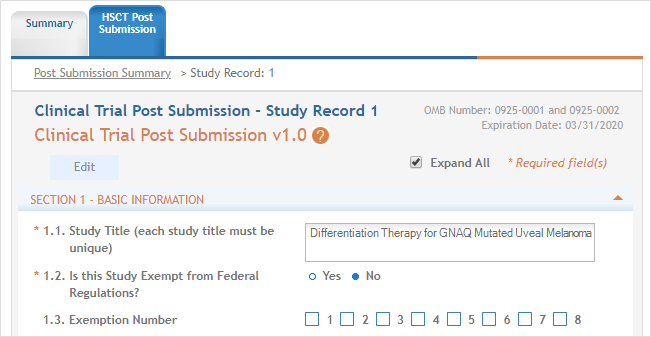
 study record is opened and the fields can be updated.
study record is opened and the fields can be updated.
Option 2
- Select the HSCT Post Submission tab and then click on the Edit button.
 (click to view)
(click to view) - Now you see that the existing study has an Edit button available and there are additional buttons to add regular or delayed onset studies.
 (click to view screen in Edit mode)
(click to view screen in Edit mode) - Select the Edit button for the existing study to open the edit screen.
 (click to view screen in Edit mode)
(click to view screen in Edit mode)
Editing Clinical Trial Information
Several fields in HSS are mapped to ClinicalTrials.gov to support clinical trial registration and reporting compliance. These fields include:
-
2.1 Conditions or Focus of Study
-
2.2 Eligibility Criteria
-
2.3 Age Limits*
-
2.6 Recruitment Status*
-
4.1 Detailed Description
-
4.1.b. Primary Purpose
-
4.1.c. Interventions
-
4.1.d. Study Phase*
-
4.1.e. Intervention Model
-
4.1.f. Masking*
-
4.1.g. Allocation*
-
4.2 Outcome Measures
-
6.1 Study Primary Completion Date*
-
6.2 Study Final Completion Date*
-
6.3 Enrollment of First Participant (Study Start) Date*
-
6.5 Reporting of Results in ClinicalTrials.gov*
*fields that are validated for congruence with ClinicalTrials.gov information
See the application guide for more information. Generally, it is best to keep ClinicalTrials.gov information up-to-date and update HSS with this information as necessitated by the FOA or the terms and conditions of the award.
Exporting HSS data for ClinicalTrials.gov registration
If HSS data entry occurred before ClinicalTrials.gov registration, the clinical trial data in HSS can be used to initiate registration in ClinicalTrials.gov. See the ASSIST Online Help, Export and Upload Data to ClinicalTrials.gov for instructions.
Using the Populate button to update clinical trial data
After a clinical trial has been registered in ClinicalTrials.gov, HSS fields that map to ClinicalTrials.gov can be updated with the information available in ClinicalTrials.gov.
-
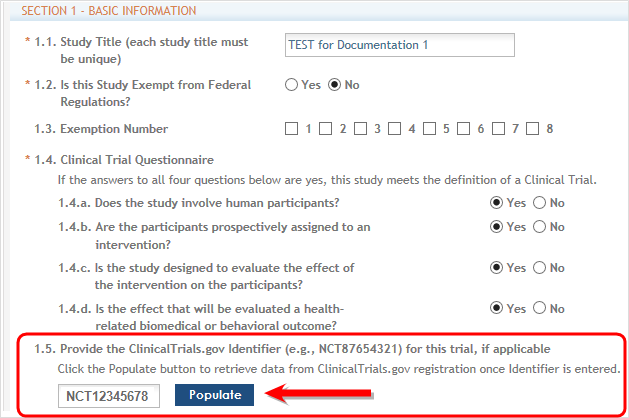
To perform this task, the ClinicalTrials.gov identifier (NCT number) should be entered in the field numbered 1.5.
-
Next, select the Populate button as shown below and the system does a best effort copy of form data from the official Clinical Trials records.
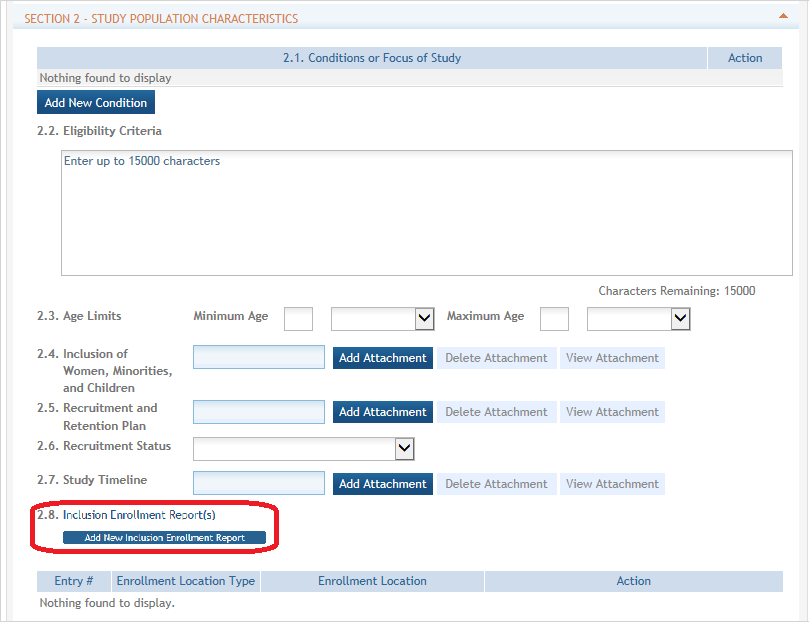
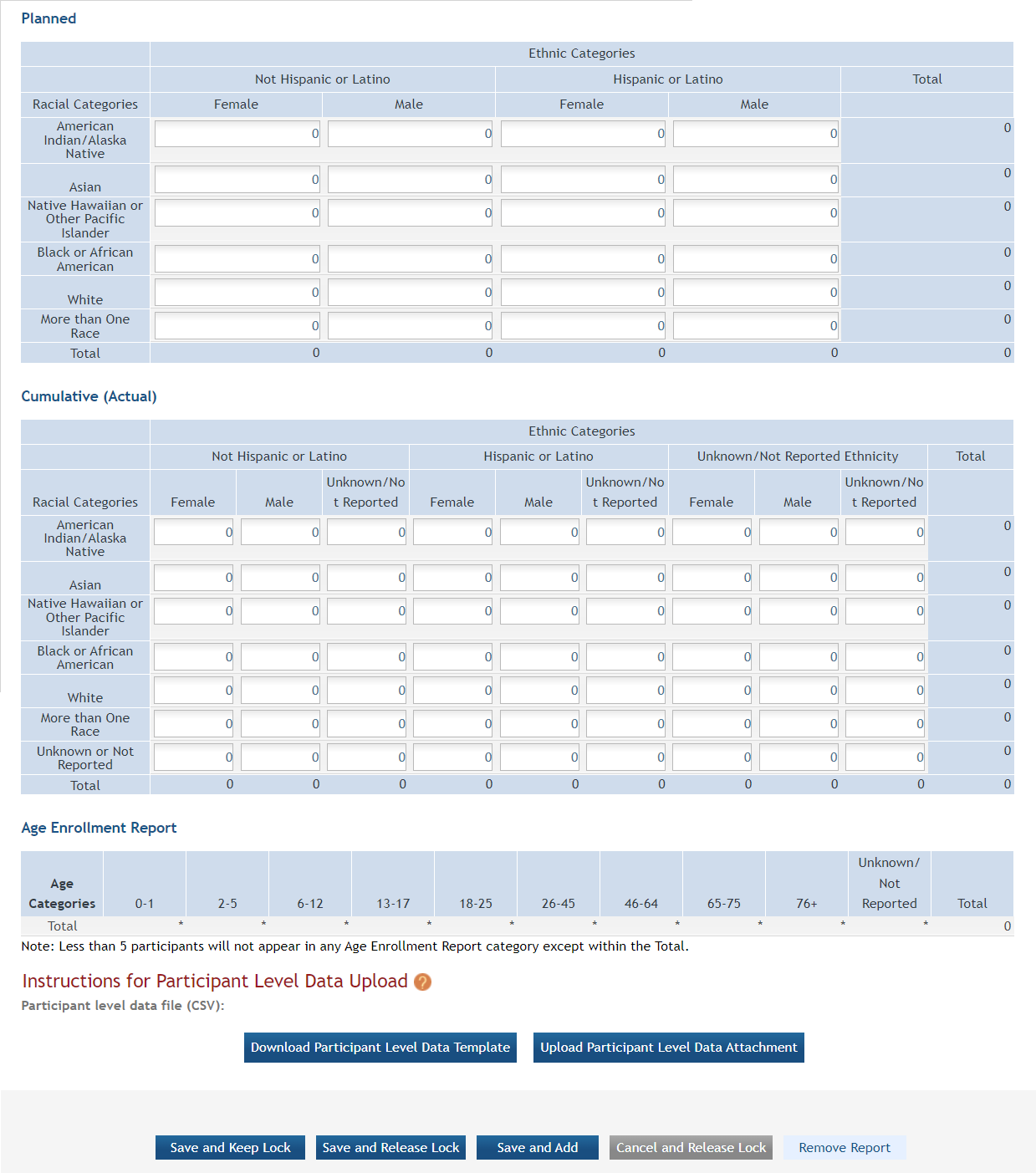
Inclusion Enrollment Report
Standalone PHS Inclusion Enrollment Report forms are no longer used. Instead, data collection for up to 20 Inclusion Enrollment Reports has been folded into each Study Record. Click on the link in Section 2.8 of the Study Record screen to initiate the Inclusion Enrollment Report. ![]() (click to view)
(click to view)
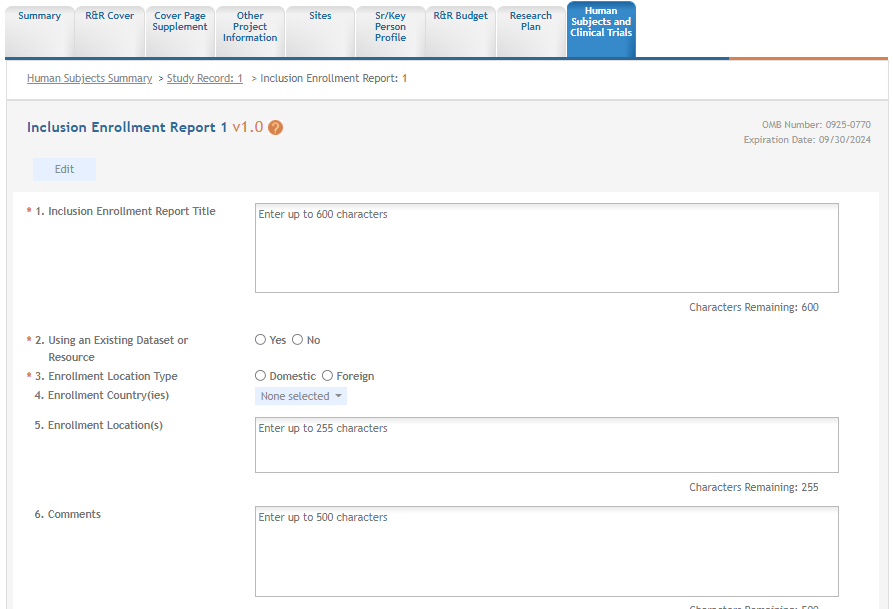
For each Inclusion Enrollment Report, applicants must create a title, and indicate whether an existing dataset or resource will be used and whether the enrollment location type is domestic or foreign.
There are also a few optional fields in the report, including a text entry Comments section. ![]() click to view top part of IER)
click to view top part of IER)
Figure 1: Top part of the Inclusion Enrollment Report screen
Planned and Cumulative enrollment data collection are in separate sections. Planned data are shown in one table. The cumulative enrollment data are displayed in separate tables for sex, race, and ethnicity information, and for the age data ![]() click to view tables)
click to view tables)
Figure 2: Bottom part of the Inclusion Enrollment Report screen
Editing Inclusion Counts
Inclusion data is found at the end of Section 2. ![]() (click to view inclusion data area)
(click to view inclusion data area)
Planned Enrollment Counts
When creating a new inclusion enrollment report that is not marked as an existing dataset or resource, planned enrollment counts are required. To add Planned counts, edit the cells in the table.
Cumulative Enrollment Counts
There are two ways to edit the existing Inclusion Enrollment Report (IER) data for Cumulative (Actual) counts:
-
You can update the cells online in the existing report itself.
-
You can provide participant-level data in a spreadsheet that populates the cumulative table after upload.
For research from competing applications with due dates prior to January 25, 2019, either method may be used.
For research from competing applications with due dates January 25, 2019 or later, participant-level data are required in progress reports (see NIH Guide Notice NOT-OD-116).
-
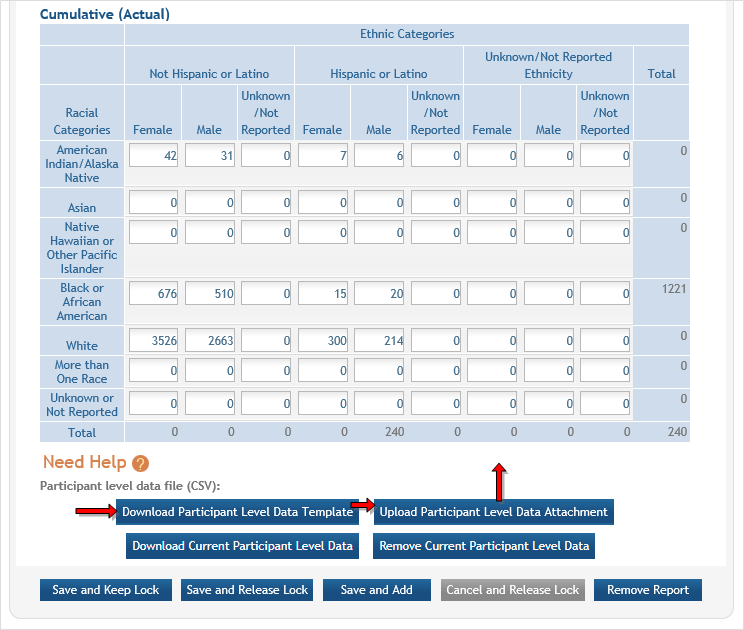
If you plan to upload the data, you must use the Participant Level Data Template. The template is a spreadsheet file in the proper CSV format to be used by the system.
-
You can download the template by selecting the Download Participant Level Data Template button. This CSV file can then be updated with new totals.
To use the template:
- Download the spreadsheet template for entering participant -level data by clicking on the Download Participant Level Data Template button. Fill the template with data for the study.
- The columns in the template should not be altered: altering the format or category titles results in an error during the uploading process.
Data can be copied/transferred into the template from another source or entered directly into the template. When copying data be sure to copy values only and ensure your data are free of formulas.
- Once the new totals have been entered into the template and the file has been saved, use the Upload Participant Level Data Attachment button to upload the file that will update the Cumulative counts.
If you need to clear the current records, use the Remove Current Participant Level Data button. ![]() (click to view Cumulative data section)
(click to view Cumulative data section)
The entire study can be previewed before submission by clicking on the Preview Study button on the left navigational column under Actions. ![]() (click to view)
(click to view)
PI and SO Actions
If the PI is making changes:
-
The PI changes the submission status to Work in Progress.
-
The PI can click the Save and Release Lock button to save the changes.
-
PI changes status to Ready for Submission.
-
SO logs into ASSIST, finds the application, and submits it.
NOTE: If the SO has delegated Submit authority to the contact PI, the PI can submit the application.
If the SO is making changes:
- The SO changes the submission status to Work in Progress.
- The SO can click the Save and Release Lock button to save the changes.
-
SO changes status to Ready for Submission.
-
The Submit action becomes active on the Application Information page.
-
SO clicks on the Submit button.
NOTE: The SO can delegate Submit authority to the contact PI. If this delegation is not done, only the SO can submit the application to NIH. The submission sends all updated study records associated with the application to NIH at one time.
Program officials and grant specialists are notified automatically of study changes and can review those changes. Some changes might require prior approval.
NOTE: If the application has been submitted and needs to be placed back into a work in progress status, refer to these instructions to perform this action; https://era.nih.gov/erahelp/ASSIST/default.htm#ASSIST_Help_Topics/5_Preview_Print_Submit/Revise_Application.htm?Highlight=status