Access Human Subjects System (HSS)
The Human Subjects System can be accessed by Principal Investigators (PIs) or Signing Officials (SOs) through either the RPPR or through the Status screen in eRA Commons
Human subjects information might need to be updated in the following scenarios:
- Post-award for updates to the Research Performance Progress Report (RPPR)
- Pre-award (post review) for just-in-time information or correction of human subjects data
- Off-cycle updates as required in the Funding Opportunity Announcement or terms and conditions of award
- Corrections to human subject data
Here is a quick summary of the ways HSS can be accessed (more detailed instructions follow):
-
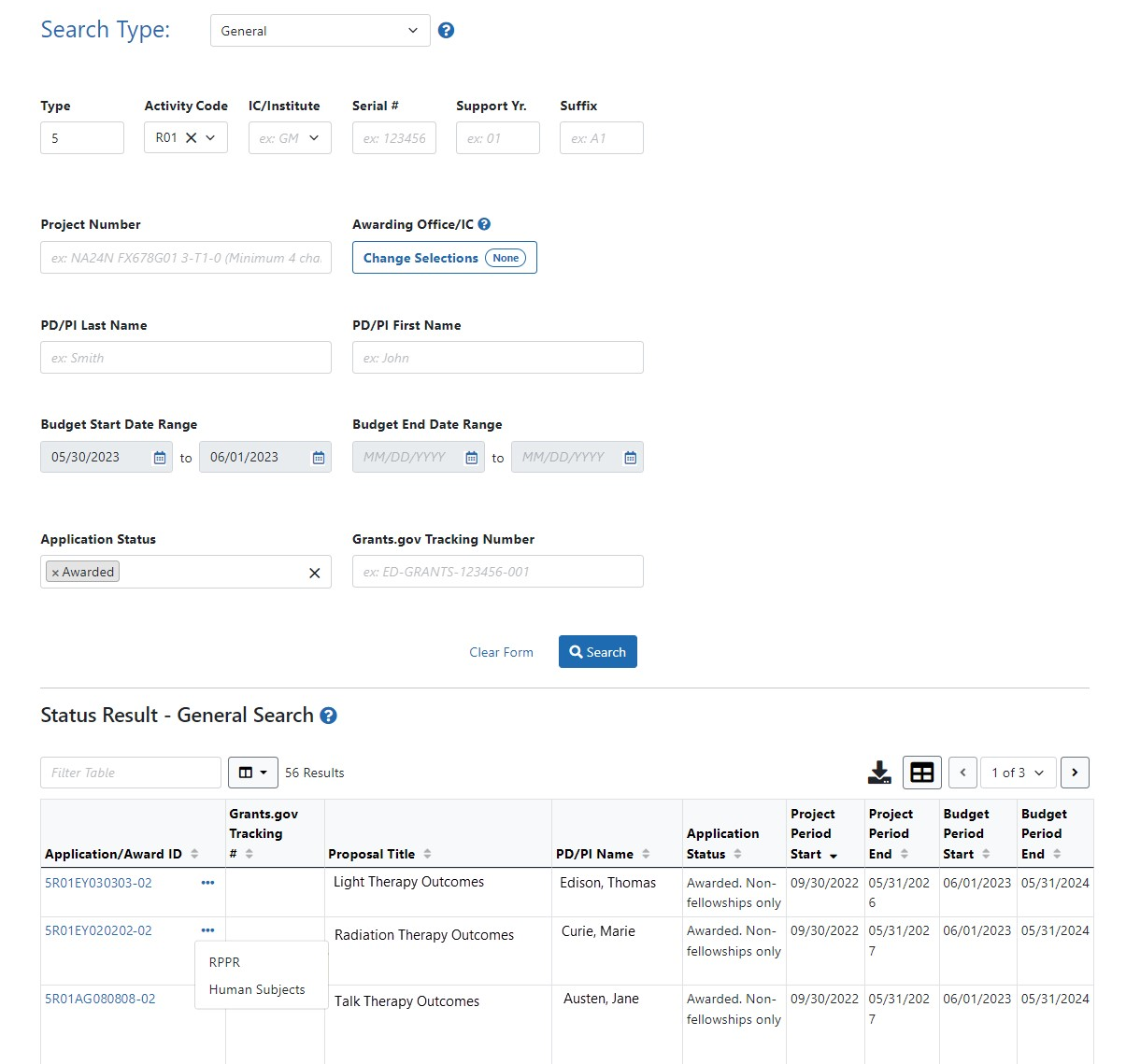
SO: Status module > General Search screen > Specific Award > three-dot ellipsis Actions button > Human Subjects link
-
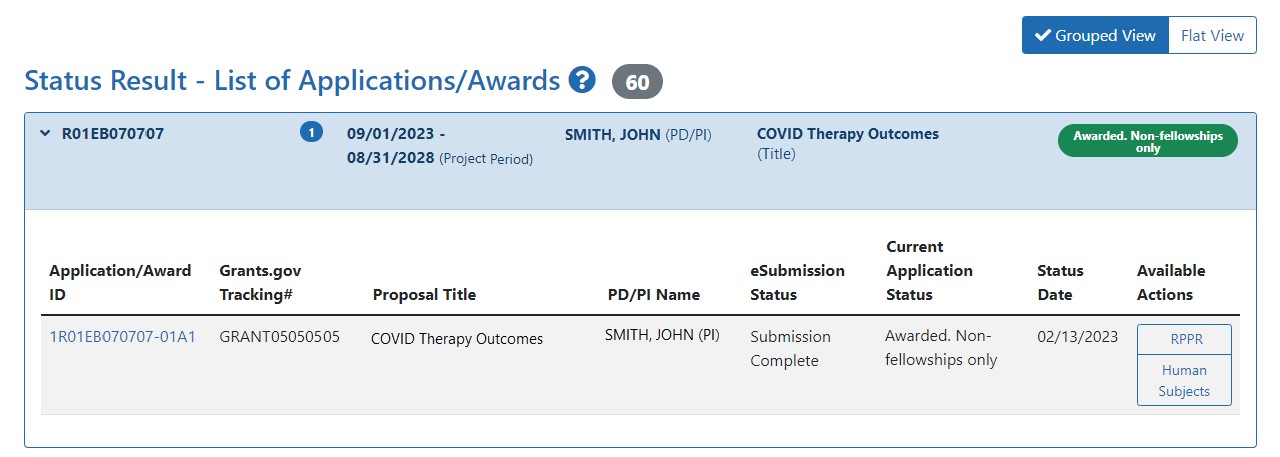
PI: Status module > List of Applications/Awards > Specific Award > Available Actions column > Human Subjects link
-
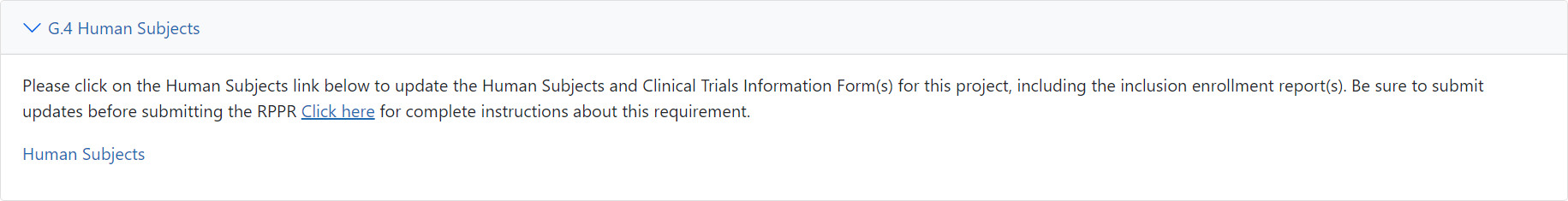
Both: RPPR tab > hyperlinked award number > G. Special Reporting Req tab > Human Subjects link
Each method results in access, via HSCT Post Submission, to information in regular study records, such as inclusion enrollment reports. Delayed onset study records are also accessible.
To edit an existing study, log into eRA Commons and access the Human Subjects link via the RPPR or Status tabs.
Access via Status
SOs
- Additionally, the SO can use the General Search on the Status screen to find a list of applications and then select the Human Subjects link in the Action column.
 (click to view Human Subjects links)
(click to view Human Subjects links)
PIs
- A PI can click the Status button, and then click List of Applications/Awards to see a list of their applications.
 (click to view)
(click to view)
- The resulting search results have a Human Subjects button in the Available Actions column on those applications with exempt or non-exempt human subjects research.
Selecting the Human Subjects button opens the Summary page for that application with the HSCT Post Submission tab available to access the human subjects data and make necessary updates, such as updates to Inclusion Enrollment Reports (IERs). IERs replace the Inclusion Data Records (IDRs) used in the prior inclusion management system. 36
Access via RPPR
To access HSS via an RPPR, select the RPPR module, click a hyperlinked award number, then click the G. Special Reporting Req tab.
After selecting the G. Special Reporting Req tab, scroll down to section G.4 Human Subjects and then select the link for Human Subjects. ![]() (click to view)
(click to view)
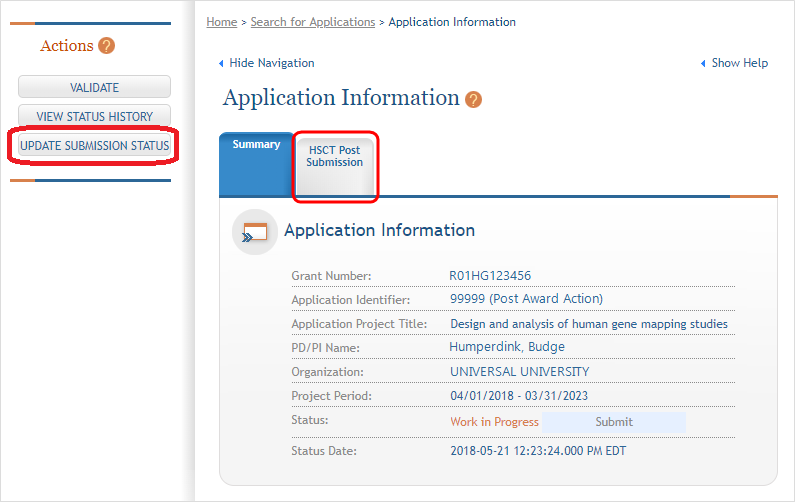
The above methods take the user to the Application Information screen and provide access to the HSCT Post Submission tab.
Click on the HSCT Post Submission tab. This takes you to a Study Record(s) screen where all study records and delayed onset studies associated with your grant are displayed. ![]() (click to view)
(click to view)
NOTE: Click the Update Submission Status button if you want to change the Status to “Work in Progress” in order to edit the application.