Study Record - Section 4 Protocol Synopsis
![]() Section 4 is the Protocol Synopsis and is only required for study records involving independent clinical trials.
Section 4 is the Protocol Synopsis and is only required for study records involving independent clinical trials.
The protocol synopsis includes:
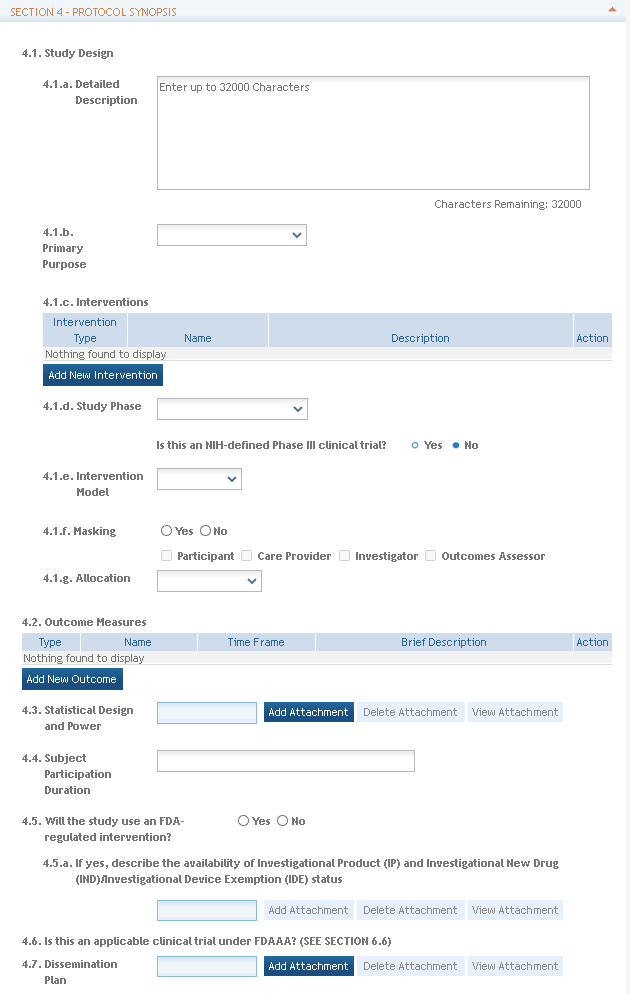
- Study Design fields related to:
- A Narrative Study Description, up to 32,000 characters
- A drop-down selection for Primary Purpose
- The type, name and description for up to 20 Interventions
- A drop-down selection for Study Phase and radio buttons to identify NIH-Defined Phase III clinical trials
- A drop-down selection for Intervention Model
- Check boxes for Masking Information. If "Yes" is selected, at least one of the related boxes must be checked.
- A drop-down selection for Allocation
- The ability to provide the name, type, time frame and description for at least one, but up to 50 Outcome Measures. Character limits for the Name and Time Frame are 255 characters and the limit for the Brief Description is 999 characters.
- A Statistical Design and Power attachment.
- A Subject Participation Duration attachment with a 255 character limit.
- Question 4.6, Is this an applicable clinical trial under FDAAA, is new. If in pre-submission, enter the answer to this question in Section 4. If in post-submission, enter this question in section 6.6. FDAAA refers to the Food and Drug Administration Amendments Act (FDAAA) of 2007. To learn more, see the Identifying an Applicable Clinical Trial under FDAAA flowchart.
- A Dissemination Plan attachment used to describe an applicant’s plan for the dissemination of NIH-funded clinical trial information and how they plan to meet the expectations of NIH’s new policies including the requirement to register and report results in ClinicalTrials.gov. Usually, one plan per application is sufficient and the plan may be attached in multiple studies.
The Description, Study Design, and Outcome Measures fields all directly map to fields in ClinicalTrials.gov.