Study Record - Section 3 Protection and Monitoring Plans
This section includes the Protection of Human Subjects attachment. Before filling the attachment out, read through the application guide instructions for the Protection of Human Subjects attachment.
All human subjects studies must provide a Protection of Human Subjects attachment and answer the question regarding multi-site studies. If applicants propose a multi-site study that will use the same protocol to conduct non-exempt Human Subject research at more than one domestic site, they need to attach your plan describing how they will comply with the NIH policy on the use of single-IRB for multi-site research. Note that the IRB Plan attachment is not required for Forms F version 2.0 and later.
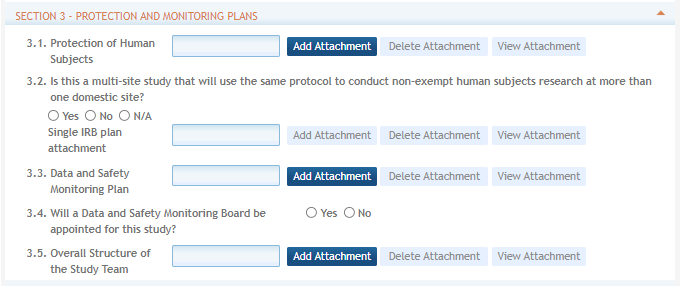
The remaining fields in this section – the Data and Safety Monitoring Plan attachment, question about the use of a Data Safety Monitoring Board, and the Overall Structure of the Study Team attachment – only apply to studies involving clinical trials, though other studies can include them if needed.
