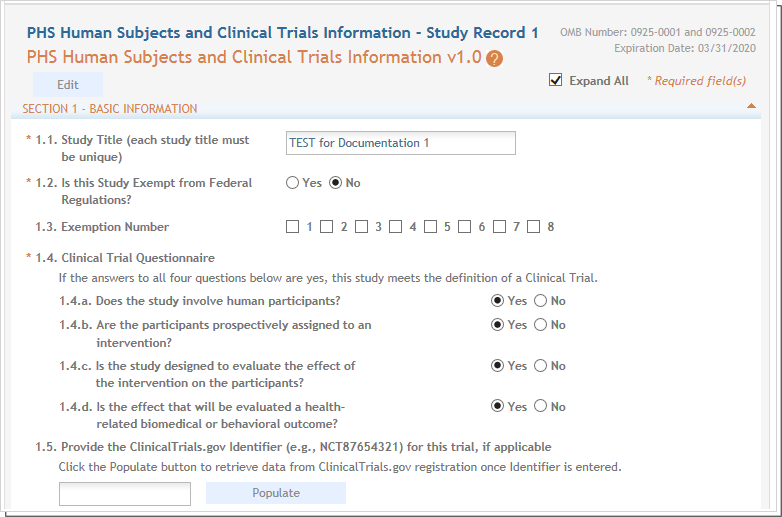
Study Record - Section 1 Basic Information
Section 1 includes basic information and must be completed for all human study records, both those with or without clinical trials. ![]() Click to view Section 1)
Click to view Section 1)
This section includes:
- A Study Title up to 600 characters, which must be unique within your organization.
- Exemption question. This is a required question and is system enforced.
- Exemption code information. Where the exemption code information provided on the Other Project Information form was for the application as a whole, this field asks about exemption code information at the study level.
- A clinical trial questionnaire. If you answered Yes to Are Human Subjects Involved? question on the Other Project Information form, then question 1.4.a Does the study involve human participants? defaults to Yes and is non-editable. If you answer Yes to all four questions, the study will be considered a clinical trial and section 5 appears on the form, requiring you to provide trial-specific data. Refer to more comprehensive instructions in the How to Apply guides located here; https://grants.nih.gov/grants/how-to-apply-application-guide.html.
- A ClinicalTrials.gov Identifier (NCT number) if available. To populate information from a trial registered at ClinicalTrials.gov, enter the NCT number in the specified format and select the Populate button. This feature will do a best effort copy of form field data as well as attachments. When the copy is done, you should check the form for completeness.
NOTE: The Human Subjects and Clinical Trials forms in ASSIST and the ClinicalTrials.gov forms have many fields in common. Collecting the NCT number in ASSIST positions us for a future exchange data with ClinicalTrials.gov to reduce data entry and provide more consistent information between systems.