Post Meeting Conflict of Interest Certification
The NIH peer review process relies on reviewers to identify any conflicts of interest (COI) that may affect the integrity of the process. The rules for identifying COIs can be found
Reviewers certify the Post Meeting Certification at the completion of a review meeting to confirm that participation in discussions about conflicted applications did not take place with the reviewer present.
The post-meeting COI certification must be electronically signed at the time reviewers complete their participation in the study section meeting. Paper certifications will no longer be accepted.
The post-meeting COI form will be available until the end of the Edit phase.
The language of the pre- and post-meeting COI certifications has been updated, so reviewers should read it carefully before certifying at the bottom of the screen. Note that the system will recognize whether it is a non-federal or federal reviewer or whether it is a grants or contracts review, and will accordingly display the right text:
To access and sign the Post Meeting Certification:
-
Access the
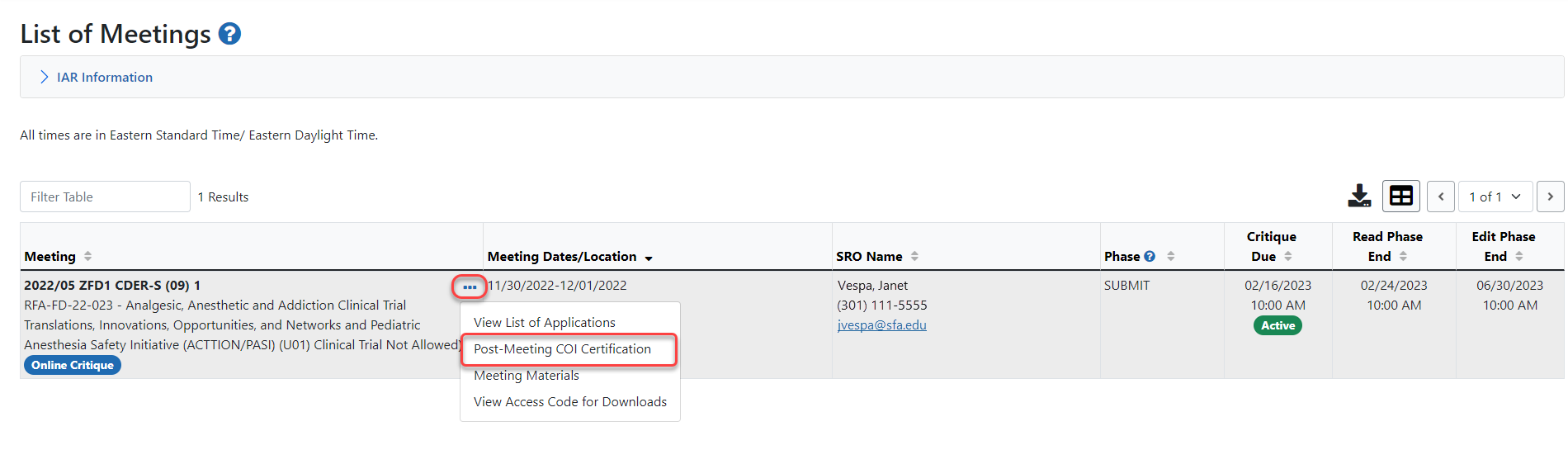
 List of Meetings screen.
List of Meetings screen. -
Select the Post-Meeting COI Certification link from the three-dot ellipses icon menu in the Meeting column of the specific meeting.
The Post Meeting Certification screen displays. The reviewer's name and address as well as the Scientific Review Group name and review date/s are at the top of the form.
At the bottom of the certification, the Printed Name of the reviewer displays along with a Signature field for capturing the electronic signature of the reviewer and the date and time of the review.
-
Optional: Select the List of Meetings link to return to the List of Meetings screen without signing the form. Selecting the Cancel button also returns the List of Meetings screen without saving the form.
-
Select the I Certify button after reading the certification.
The Signature field updates with the electronic signature of the reviewer and timestamp. If necessary, reviewers may access the form and follow the steps for submission to re-submit the certification.
 Click here for an example of the screen. Note that there is some additional text in the Federal Reviewer version that is not part of the Non-Federal Reviewer version. The format, rules and final certification paragraph contain the same language and links.
Click here for an example of the screen. Note that there is some additional text in the Federal Reviewer version that is not part of the Non-Federal Reviewer version. The format, rules and final certification paragraph contain the same language and links.A list of examples for Federal, Non-Federal grants and contract Post-Meeting Certifications can be found at the links below:
Grants certifications (effective 05/25/2022)
- NIH Conflict of Interest Grants Post-Review Certification, Non-Federal (effective 05/19/2022)
- NIH Conflict of Interest Grants Post-Review Certification, Federal (effective 05/19/2022)
Contracts certifications (effective 05/25/2022)
- NIH Conflict of Interest Contract Post-Review Certification, Non-Federal (05/19/2022)
- NIH Conflict of Interest Contract Post-Review Certification, Federal (05/19/2022)
-
Select the List of Meetings link to exit the certification.
NOTE: In rare cases, the I Certify button may not appear on the screen. This is due to multiple profiles in the system for the same person. Contact the SRO* or eRA Service Desk in this case.
NOTE: If the post-meeting COI has not been signed and the meeting end date has been passed, a reminder email will be sent to the MLG email address indicating the relevant meeting name and date as well as the date when access to the meeting will expire.
TIP: *Other Transaction Authority (OTA) — Some screens and terminology may differ to accommodate review of OTA, a type of award that is neither a grant nor a contract but a different way of funding that is used across NIH. These changes will typically not be visible to NIH or agency reviewers.