Details on Certificate of Confidentiality Eligibility Questions
The Online Certificate of Confidentiality System includes questions to determine if a research project may be eligible to receive a Certificate of Confidentiality (CoC) issued by NIH. The questions asked are partly derived from United States Code (laws) or the Code of Federal Regulations (explanations of how agencies carry out laws).
NOTE:
- Non-federally-funded research might also qualify to receive a CoC from NIH.
- The NIH Guide Notice for NIH Policy for Issuing Certificates of Confidentiality discusses identifiable, sensitive information, as well as human subject research, in detail.
After selecting a funding source and clicking Next, the Certification questions display.
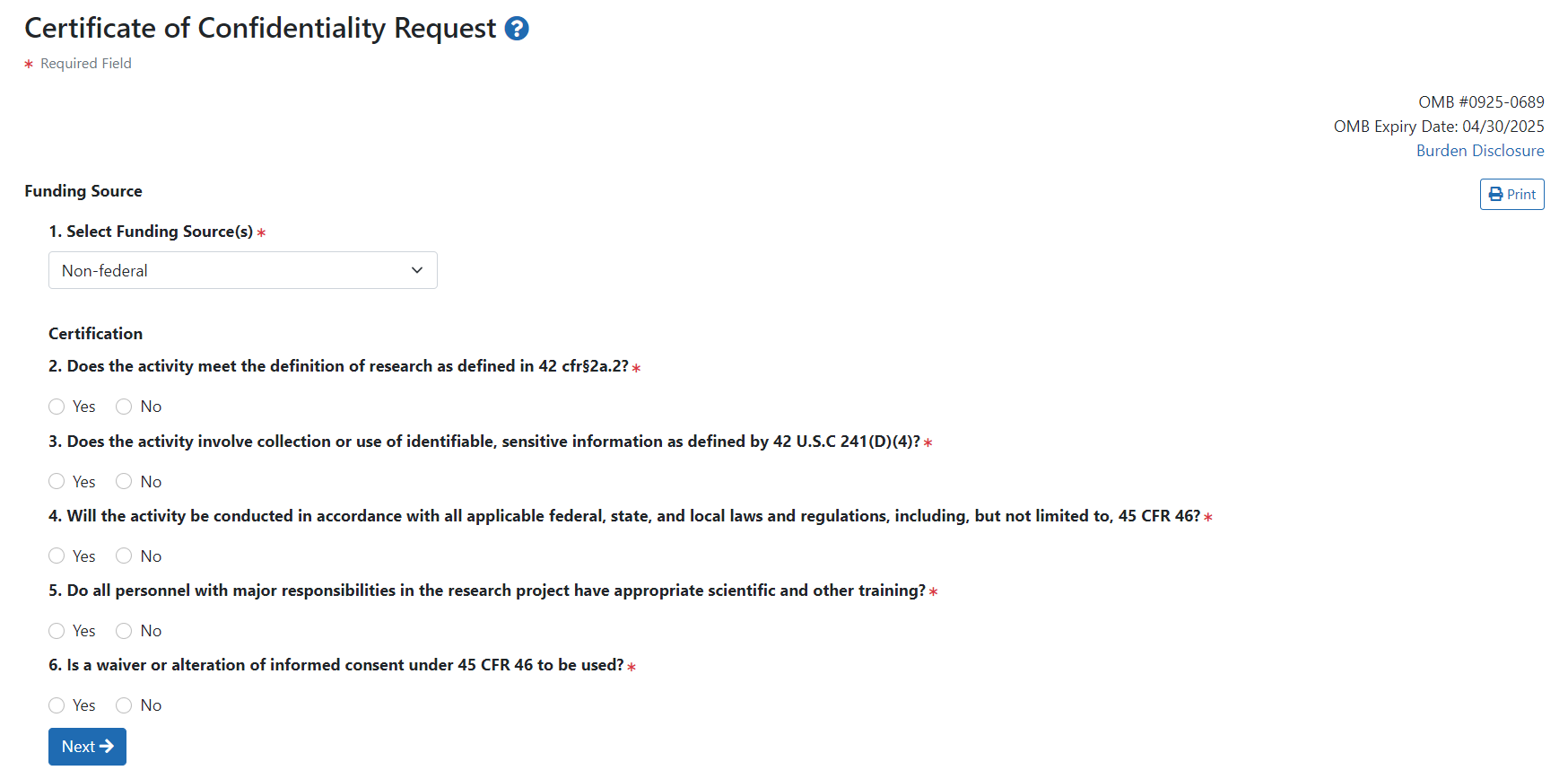
See the questions below to find out more about each question:
The only answers to this question that will continue the Online CoC request are Other DHHS agency --> Other, Other federal agency --> Other, or Non-federal.
DO NOT use the Online Certificate of Confidentiality System if your research is funded by the NIH. NIH-funded research studies that are within the scope of the NIH CoC Policy are automatically deemed issued a CoC
In addition, the Online Certificate of Confidentiality System is not the method used to request a CoC for all federal agencies. If your research is funded by any of the following, the Online Certificate of Confidentiality System will display an alert message that directs you to CoC information for the respective agency.
HHS agencies and federal departments that have their own process for issuing a Certificate of Confidentiality:
- AHRQ - Agency for Healthcare Research & Quality
- CDC - Centers for Disease Control and Prevention
- FDA - Food and Drug Administration
- HRSA - Health Resources and Services Administration
- IHS - Indian Health Service
- SAMHSA - Substance Abuse and Mental Health Services Administration
- BARDA – Biomedical Advanced Research and Development Authority
- DOJ - Department of Justice
For research funded by agencies listed above, see the non-NIH HHS Agencies CoC Coordinators and Contact information on the NIH website for CoC Coordinator contact information at the applicable funding agency.
NOTE: BARDA funded research studies with awards issued prior to July 17, 2023, are eligible to request a CoC from NIH.
Clicking Yes on this question is consistent with eligibility for a CoC.
This refers to the Title 42 of the Code of Federal Regulations (Public Health).
The relevant definition from the code is:
Research means systematic study directed toward new or fuller knowledge and understanding of the subject studied. The term includes, but is not limited to, behavioral science studies, surveys, evaluations, and clinical investigations.
Clicking Yes on this question is consistent with eligibility for a CoC.
This refers to Title 42 of the United States Code (Public Health and Welfare).
The relevant definition from the code is:
(A) An individual is identified; or
(B) There is at least a very small risk, as determined by current scientific practices or statistical methods, that some combination of the information, the request, and other available data sources could be used to deduce the identity of an individual
Clicking Yes on this question is consistent with eligibility for a CoC.
This question asks you to verify that the research activity complies with all federal, state, and local laws and regulations, including Title 45 (Public Welfare) Part 46 (Protection of Human Subjects) of the Code of Federal Regulations, located at the following website:
https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-A/part-46
Clicking Yes on this question is consistent with eligibility for a CoC.
This question ascertains if personnel who have major research responsibilities also possess the training necessary to perform the research.
Clicking No on this question is consistent with eligibility for a CoC. However, clicking Yes results in a follow up question to further determine eligibility.
This question asks if the IRB waived or altered the requirement to obtain the informed consent (i.e., you are not obtaining informed consent or parental permission), as described in Title 45 (Public Welfare) Part 46 (Protection of Human Subjects) of the Code of Federal Regulations. If you are obtaining written or oral informed consent from participants or their legally authorized representatives, answer No to this question.
See section §46.116 for a description of informed consent.
NOTE: Do not answer YES to this question if the IRB approved the plan for the investigator to obtain information or specimens for the purpose of screening, recruiting, or determining eligibility of prospective participants without informed consent of the participant or participants legally authorized representative under 45 CFR 46.116(g) OR if the IRB waived the requirement to obtain a signed informed consent form under 45 CFR 46.117(c) (i.e., the IRB waived documentation of informed consent).
If you are using a waiver or alteration, answer Yes and another question appears.
Clicking Yes on this question is consistent with eligibility for a CoC. Clicking Yes means the waiver or alteration of informed consent has been approved by the reviewing Institutional Review Board (IRB).
This question appears only if you answered Yes to question 6. Details on obtaining a waiver or alteration of consent are contained in Title 45 (Public Welfare) Part 46 (Protection of Human Subjects) of the Code of Federal Regulations, section §46.116.
US Code §46.116, sections (e) and (f), titled, Waiver or alteration of consent in research involving public benefit and service programs conducted by or subject to the approval of state or local officials, and General waiver or alteration of consent, provide details on waiving the requirement to obtain informed consent or altering or omitting some or all of the elements of informed consent, including IRB findings and approval.
See section §46.116 section (e) and section §46.116 section (f):
After answering all questions, click the Next button to continue.
