If your answers to eligibility questions determine that your research is eligible for a Certificate of Confidentiality (CoC), you will see a data entry screen after answering eligibility questions and clicking the Next button. Here, you enter the details of your research project, contact info, and drug administration details.
All fields with a red asterisk are required. Toggle the sections on the page to expand or collapse by clicking the View All / Hide All toggle button at the right. Click the Print button at the right of the page to display a printer-friendly form with all questions and answers entered thus far.
There are four main sections to the Certificate of Confidentiality Request screen, and two ways to submit:
You must enter the project details before completing other sections of this form; otherwise you won't be able to save performance sites, key personnel, or drugs later in the form.
In the ![]() top section titled Project Details, enter the following:
top section titled Project Details, enter the following:
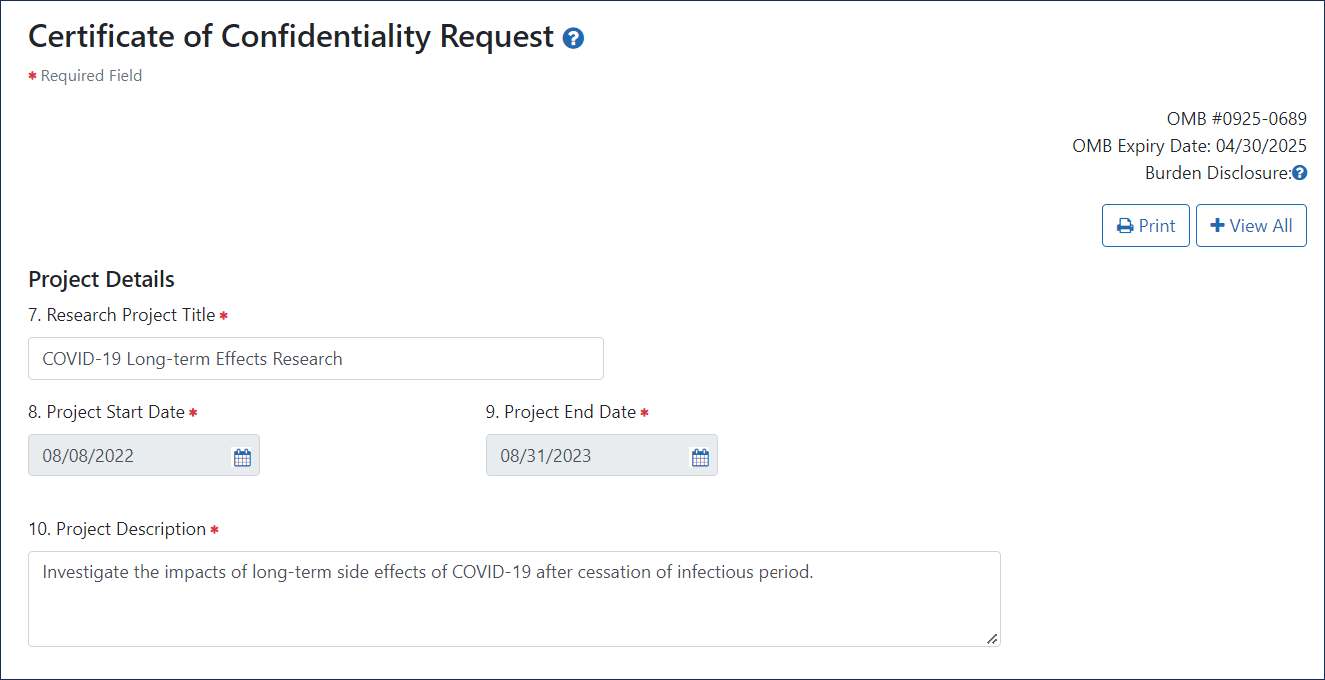
- 7. Research Project Title.
- 8. Project Start Date. This date must be in the future. If the research has already begun, enter today's date plus one business day. If the research has not yet begun, enter its future start date.
- 9. Project End Date.
- 10. Project Description. Include enough detail to show that the research project falls within the health-related missions of NIH or HHS. This field is limited to 1000 characters.
If the requesting institution is not in the United States, then at least one of the performance sites must be in the United States to proceed. If you have questions about this requirement, contact the NIH CoC Coordinator at .
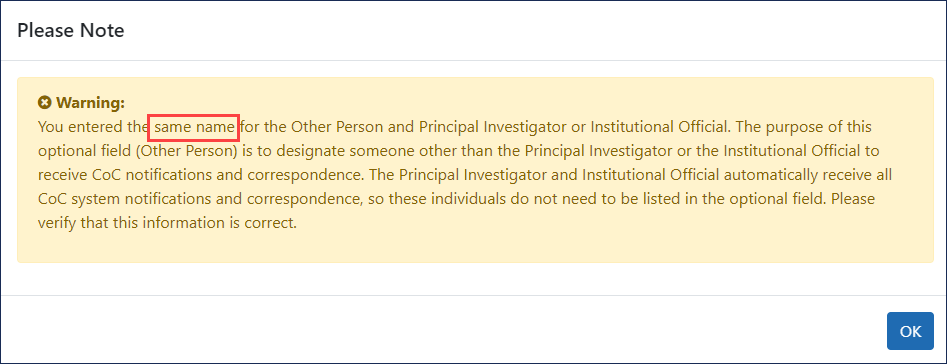
Also, you will receive a warning if the institutional official (IO) and the principal investigator (PI) are the same person or share the same email address. Go here to view the FAQ about institutional officials. If this information is accurate, you may proceed through this warning.
In the ![]() Institution and Performance Site Details section, enter the following:
Institution and Performance Site Details section, enter the following:
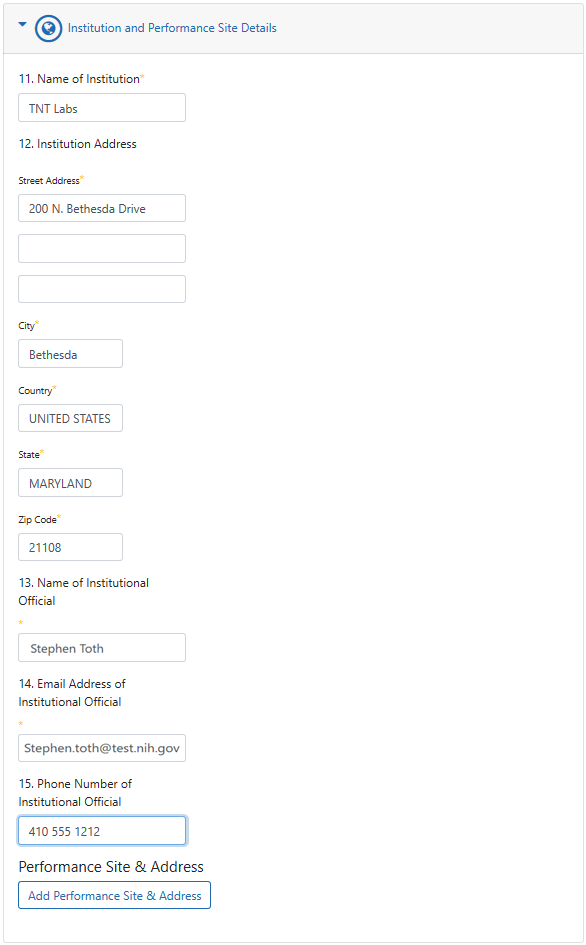
- 11. Name of Institution. The institution that will be conducting the research project. For multisite projects in which the coordinating center or lead site is requesting a Certificate on behalf of all member institutions (e.g., participating sites), enter the requesting institution name.
- 12. Institution Address.
NOTE: The State field is disabled unless "UNITED STATES" or other applicable country is entered as the country. Also, you must use the dropdown menu to specify State and Country rather than typing the state or country. Not doing so might result in a submittal error.
- 13. Name of Institutional Official. The authorized institutional official (IO) is the individual named by the requesting institution who is authorized to act for that institution and assumes on behalf of the institution the obligations imposed by the Certificate of Confidentiality as well as obligations imposed by the Federal laws, regulations, and other requirements. The IO must have signature or other authority to submit the request.
- 14. Email Address of Institutional Official. After you submit the CoC request, the email address you enter for the IO will receive an email with a link to the original request. The IO clicks the link, reviews the request, and agrees to a set of legal obligations imposed by the CoC. This email address will receive all further communications on this CoC, so be sure that this email address is correct and routinely monitored.
- 15. Phone Number of Institutional Official.
Next you add one or more performance sites. If there is only one performance site, and it is the same as the institution requesting the CoC, then you can skip entering information in this field. Click the Add Performance Site & Address button to display ![]() fields for entering performance site data. Then enter the following:
fields for entering performance site data. Then enter the following:
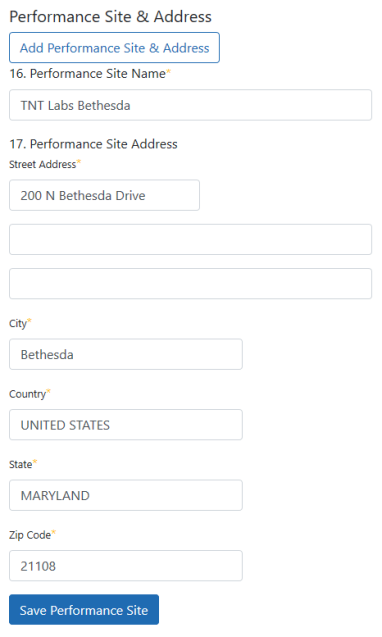
- 16. Performance Site Name. Name of institution or other identifier where the research will mainly take place. For multisite projects, enter the name(s) of all member institution(s) (e.g., participating sites).
- 17. Performance Site Address. Physical address where research will mainly take place. For multisite projects, enter the physical address(es) of all member institution(s) (e.g., participating sites).
NOTE: The State field is disabled unless "UNITED STATES" or other applicable country is entered as the country. Also, you must use the dropdown menu to specify State and Country rather than typing the state or country. Not doing so might result in a submittal error.
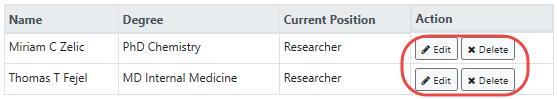
Click the Save Performance Site button when finished. For multisite projects, click the Add Performance Site & Address repeatedly to enter the name(s) of all member institution(s) (e.g., participating sites). A table displays, listing the performance sites that you have added. You can edit or delete individual performance site information by clicking the Edit and Delete buttons in the Action column of the table: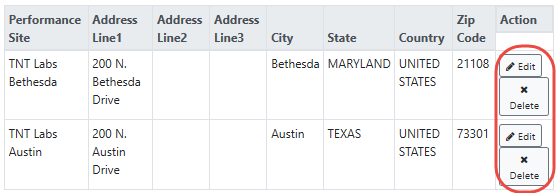
In the ![]() Principal Investigator and Other Key Personnel section, enter the following:
Principal Investigator and Other Key Personnel section, enter the following:
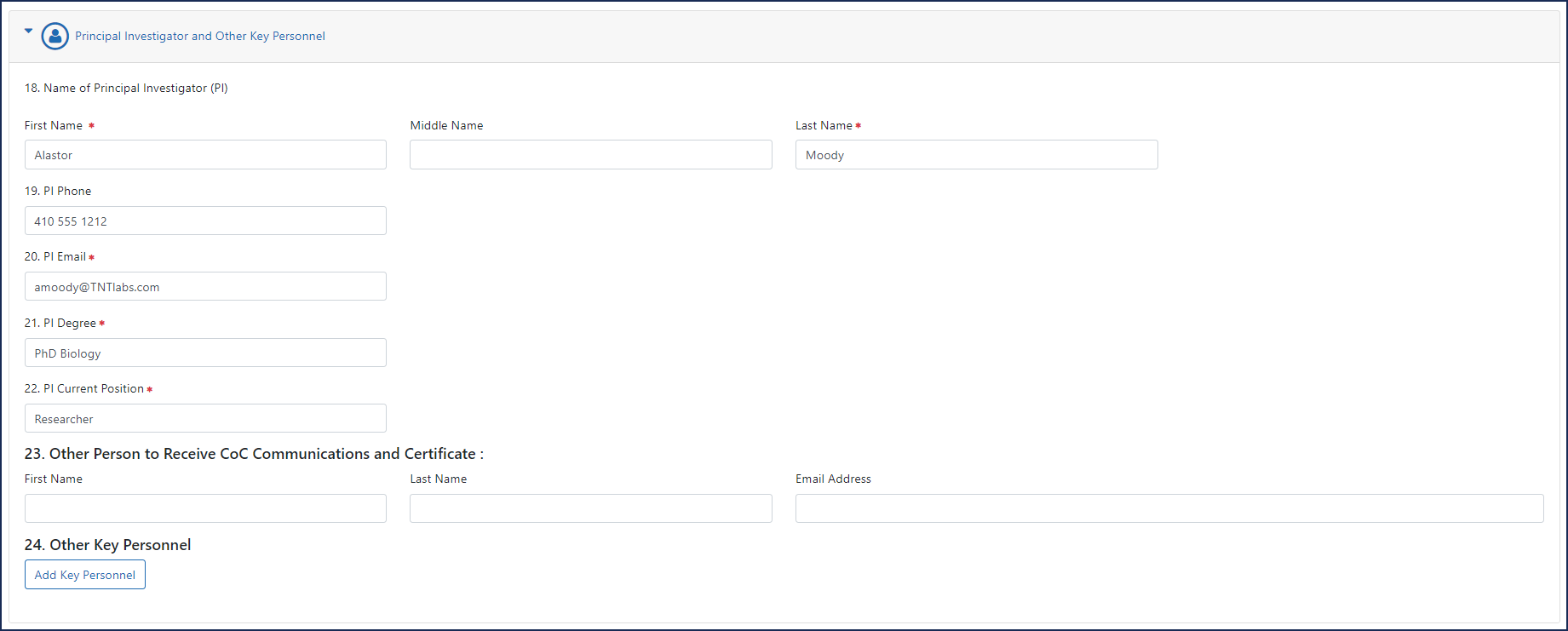
- 18. Name of Principal Investigator (PI). The person leading the research team.
- 19. PI Phone.
- 20. PI Email. The PI will receive a notification when the IO submits the CoC request to NIH. See Confirmation Email.
- 21. PI Degree. Enter the terminal degree of the PI.
- 22. PI Current Position. List the PI's title at the institution.
- 23. Other Person to Receive CoC Communications and Certificate.List another individual who should receive CoC information related to your research project. The purpose of this item is to designate a person in addition to the IO and PI to receive CoC notifications and correspondence.
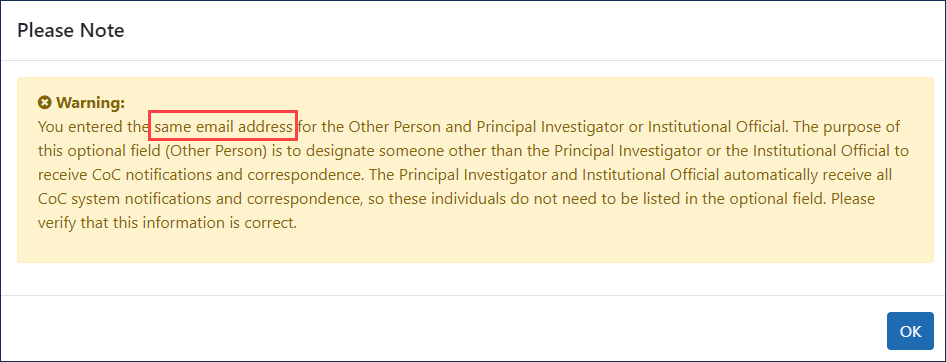
If you list the same email address for this section as either the IO or the PI, a ![]() same-email warning appears.
same-email warning appears.
NOTE: Item 23 is optional, but if you choose to list another person, you must complete all three fields (First Name, Last Name, Email Address).
- 24. Other Key Personnel. Click the Add Key Personnel button to display fields where you can record key personnel name, degree, and position. Key personnel are individuals who contribute to the scientific development or execution of a project in a substantive, measurable way, whether or not they receive salaries or compensation.
If drugs will be administered as part of the research, do the following in the ![]() Administration of Drugs section:
Administration of Drugs section:
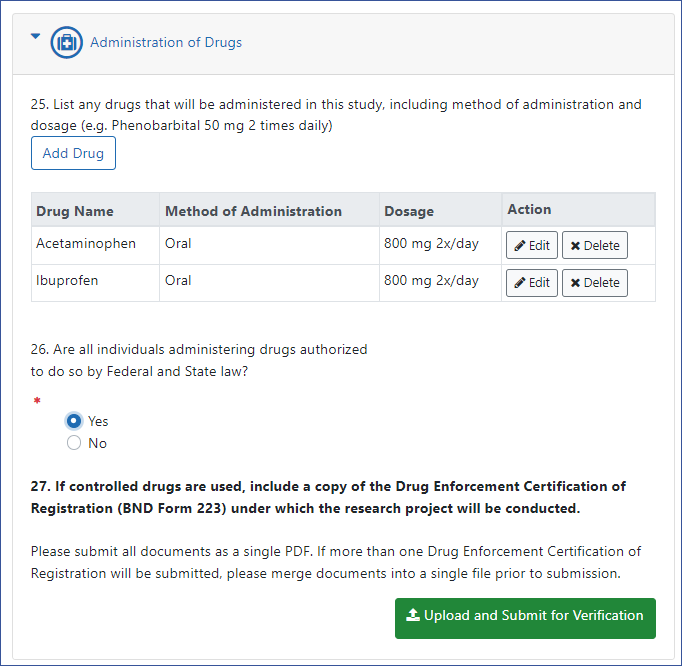
- Click the Add Drug button and enter the following:
- Name of Drug. Example: ibuprofen
- Method of Administration. Example: oral administration
- Dosage. Example: 600 mg 2x/day
- After entering a drug, click the Save Drug button. Click Add Drug additional times to enter each drug that will be administered in the study. A table appears, listing drugs that you have added. You can edit or delete individual drug information by clicking the Edit or Delete buttons in the Action column of the table.
- After you are done adding drug(s), answer Yes or No to "Are all individuals administering drugs authorized to do so by Federal and State law?" Answering No will result in a message indicating ineligibility for a CoC.
- For item 27, if one or more drugs being administered in your study are a controlled drug, you must include a PDF copy of the Drug Enforcement Certification of Registration (BND Form 223) under which the research project will be conducted. If you need to upload multiple certification forms, merge them into one PDF file. This file must be under 6 MB in size. Merging and compression can be done with Adobe Acrobat. Do not upload until you are ready to submit the request; see Methods to Submit the CoC Request.
There are two ways to submit the CoC request: The Upload and Submit Verification button or Submit for Verification button:
- Use the Upload and Submit for Verification button if there are one or more controlled drugs that will be administered in your research project, which lets you upload your drug enforcement certification of registration form(s).
- Use the Submit for Verification button if there are no controlled drugs that will be administered in your research project.
To Submit the CoC Request for Verification:
- Before you click on a Submit for Verification button, carefully check the information you have entered and make corrections, if needed.
- Print the CoC request for your records using the Print button on the top right of the web page.
- Click a Submit button:
- If you don't need to upload a Certification, click the Submit for Verification button at the bottom of the page:

- If you need to upload a Certification, click the Upload and Submit for Verification button (found under the Administration of Drugs section):

In the Confirmation Needed dialog, click the Yes button.In the Confirmation Needed dialog, click the Yes Proceed with Upload button, then browse to select the Certification PDF.
- If you don't need to upload a Certification, click the Submit for Verification button at the bottom of the page:
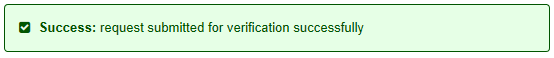
- In the browser, you will see a success message:
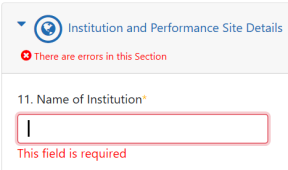
If there are errors on the form, the form is not submitted, and you can scroll through the form to see the errors in red and correct:
IMPORTANT: After submitting for verification, the CoC request is NOT final. The individual identified as the institutional official will receive an email with a link to the requestor's submitted data and must verify the data and confirm legal obligations imposed by the CoC. Once this is done, the institutional official must click the button to submit the CoC request to NIH. See Verifying a Certificate of Confidentiality for details on the institutional official verification process.